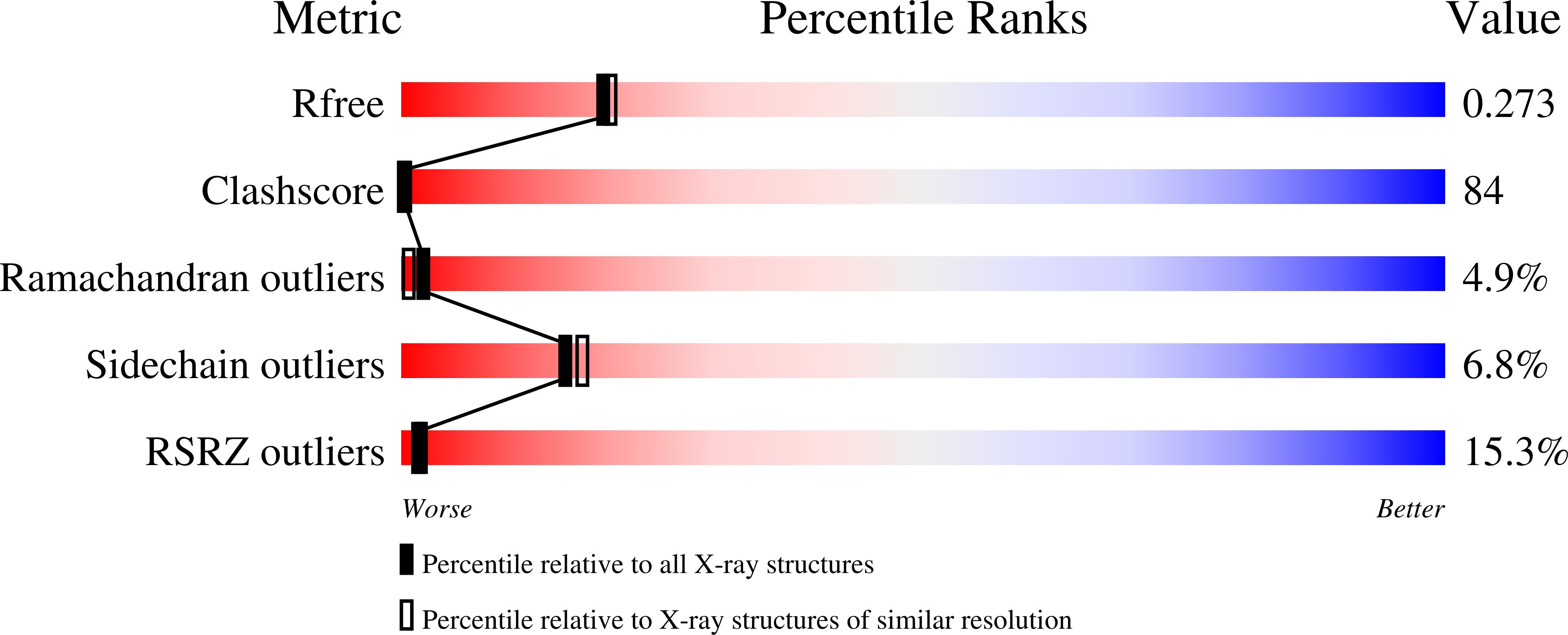
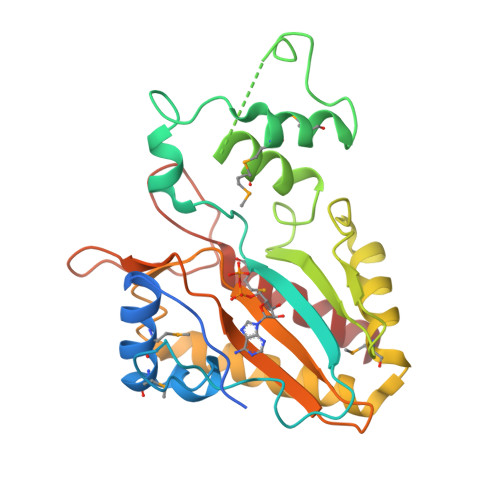
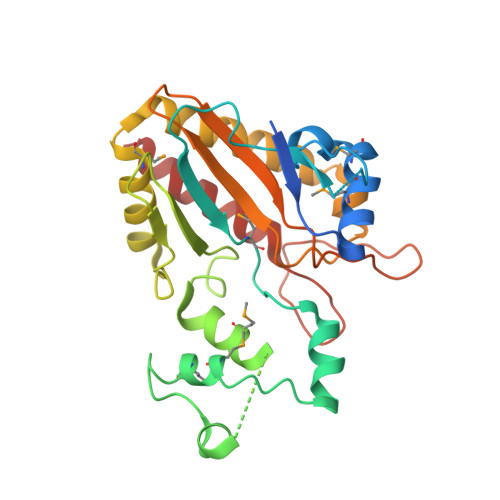
Crystal structure of the catalytic core of inositol 1,4,5-trisphosphate 3-kinase
Miller, G.J., Hurley, J.H.(2004) Mol Cell 15: 703-711
- PubMed: 15350215
- DOI: https://doi.org/10.1016/j.molcel.2004.08.005
- Primary Citation of Related Structures:
1TZD - PubMed Abstract:
Soluble inositol polyphosphates are ubiquitous second messengers in eukaryotes, and their levels are regulated by an array of specialized kinases. The structure of an archetypal member of this class, inositol 1,4,5-trisphosphate 3-kinase (IP3K), has been determined at 2.2 angstroms resolution in complex with magnesium and adenosine diphosphate. IP3K contains a catalytic domain that is a variant of the protein kinase superfamily, and a novel four-helix substrate binding domain. The two domains are in an open conformation with respect to each other, suggesting that substrate recognition and catalysis by IP3K involves a dynamic conformational cycle. The unique helical domain of IP3K blocks access to the active site by membrane-bound phosphoinositides, explaining the structural basis for soluble inositol polyphosphate specificity.
Organizational Affiliation:
Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, MD 20892 USA.