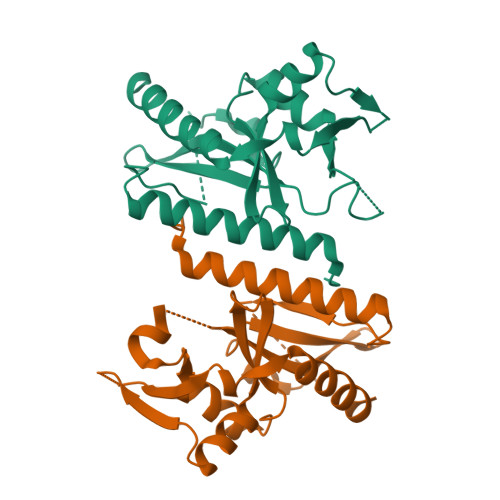
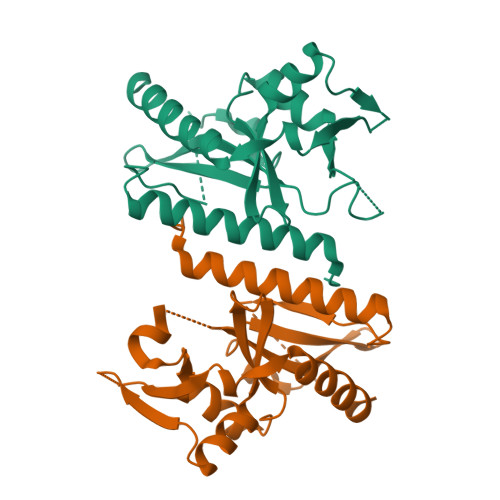
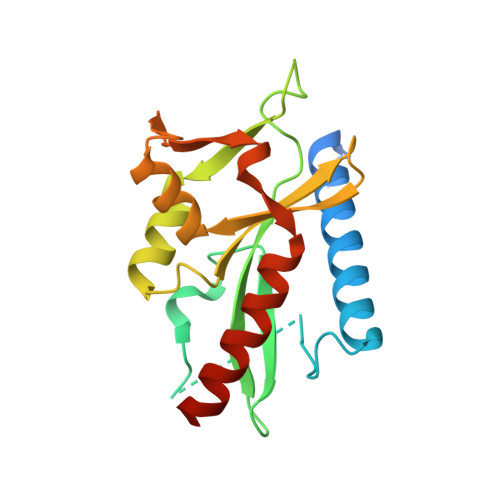
The crystal structure of D212 from sulfolobus spindle-shaped virus ragged hills reveals a new member of the PD-(D/E)XK nuclease superfamily.
Menon, S.K., Eilers, B.J., Young, M.J., Lawrence, C.M.(2010) J Virol 84: 5890-5897
- PubMed: 20375162
- DOI: https://doi.org/10.1128/JVI.01663-09
- Primary Citation of Related Structures:
2W8M - PubMed Abstract:
Structural studies have made significant contributions to our understanding of Sulfolobus spindle-shaped viruses (Fuselloviridae), an important model system for archaeal viruses. Continuing these efforts, we report the structure of D212 from Sulfolobus spindle-shaped virus Ragged Hills. The overall fold and conservation of active site residues place D212 in the PD-(D/E)XK nuclease superfamily. The greatest structural similarity is found to the archaeal Holliday junction cleavage enzymes, strongly suggesting a role in DNA replication, repair, or recombination. Other roles associated with nuclease activity are also considered.
Organizational Affiliation:
Thermal Biology Institute, Montana State University, Bozeman, MT 59717, USA.