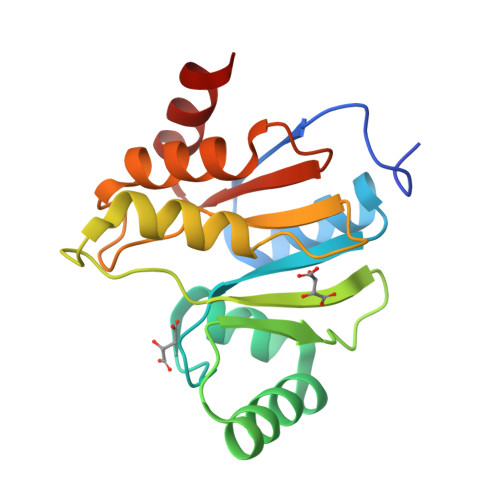
Crystal structures of the X-domains of a Group-1 and a Group-3 coronavirus reveal that ADP-ribose-binding may not be a conserved property.
Piotrowski, Y., Hansen, G., Boomaars-van der Zanden, A.L., Snijder, E.J., Gorbalenya, A.E., Hilgenfeld, R.(2009) Protein Sci 18: 6-16
- PubMed: 19177346
- DOI: https://doi.org/10.1002/pro.15
- Primary Citation of Related Structures:
3EJF, 3EJG, 3EKE - PubMed Abstract:
The polyproteins of coronaviruses are cleaved by viral proteases into at least 15 nonstructural proteins (Nsps). Consisting of five domains, Nsp3 is the largest of these (180-210 kDa). Among these domains, the so-called X-domain is believed to act as ADP-ribose-1''-phosphate phosphatase or to bind poly(ADP-ribose). However, here we show that the X-domain of Infectious Bronchitis Virus (strain Beaudette), a Group-3 coronavirus, fails to bind ADP-ribose. This is explained on the basis of the crystal structure of the protein, determined at two different pH values. For comparison, we also describe the crystal structure of the homologous X-domain from Human Coronavirus 229E, a Group-1 coronavirus, which does bind ADP-ribose.
Organizational Affiliation:
Institute of Biochemistry, Center for Structural and Cell Biology in Medicine, University of Lübeck, Ratzeburger Allee 160, 23538 Lübeck, Germany.