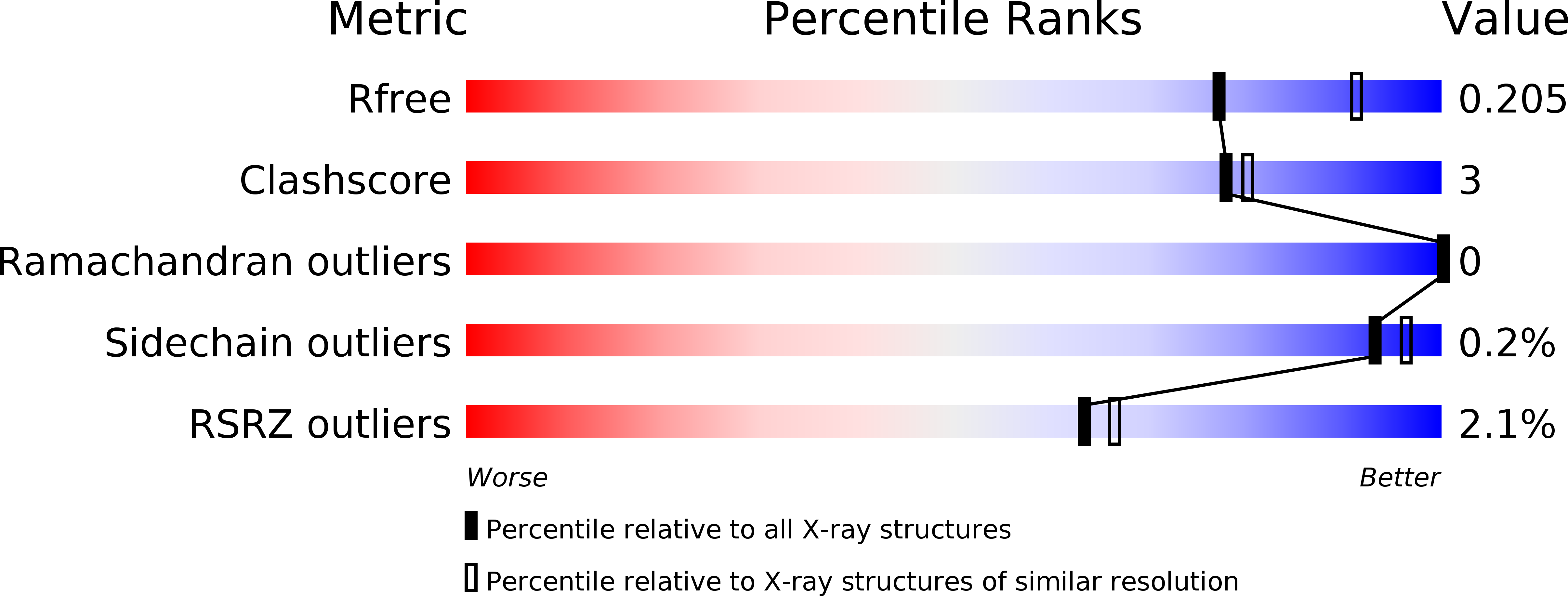
X-ray crystal structure of Vibrio alkaline phosphatase with the non-competitive inhibitor cyclohexylamine.
Asgeirsson, B., Markusson, S., Hlynsdottir, S.S., Helland, R., Hjorleifsson, J.G.(2020) Biochem Biophys Rep 24: 100830-100830
- PubMed: 33102813
- DOI: https://doi.org/10.1016/j.bbrep.2020.100830
- Primary Citation of Related Structures:
6T26 - PubMed Abstract:
Para -nitrophenyl phosphate, the common substrate for alkaline phosphatase (AP), is available as a cyclohexylamine salt. Here, we report that cyclohexylamine is a non-competitive inhibitor of APs. Cyclohexylamine inhibited four different APs. Co-crystallization with the cold-active Vibrio AP (VAP) was performed and the structure solved. Inhibition of VAP fitted a non-competitive kinetic model (K m unchanged, V max reduced) with IC 50 45.3 mM at the pH optimum 9.8, not sensitive to 0.5 M NaCl, and IC 50 27.9 mM at pH 8.0, where the addition of 0.5 M NaCl altered the inhibition to the level observed at pH 9.8. APs from E. coli and calf intestines were less sensitive to cyclohexylamine, whereas an Antarctic bacterial AP was similar to VAP in this respect. X-ray crystallography at 2.3 Å showed two binding sites, one in the active site channel and another at the surface close to dimer interface. Antarctic bacterial AP and VAP have Trp274 in common in their active-sites, that takes part in binding cyclohexylamine. VAP variants W274A, W274K, and W274H gave IC 50 values of 179 mM, 188 mM and 187 mM, respectively, at pH 9.8. The binding of cyclohexylamine in locations at the dimeric interface and/or in the active site of APs may delay product release or reduce the rate of catalytic step(s) involving conformational changes and intersubunit communications. Cyclohexylamine is a common chemical in industries and used as a counterion in substrates for alkaline phosphatase, a clinically important and common enzyme in the biosphere.
Organizational Affiliation:
Department of Biochemistry, Science Institute, University of Iceland, Dunhagi 3, 107 Reykjavik, Iceland.