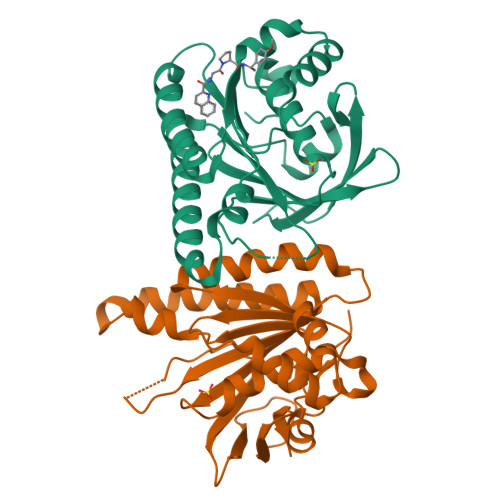
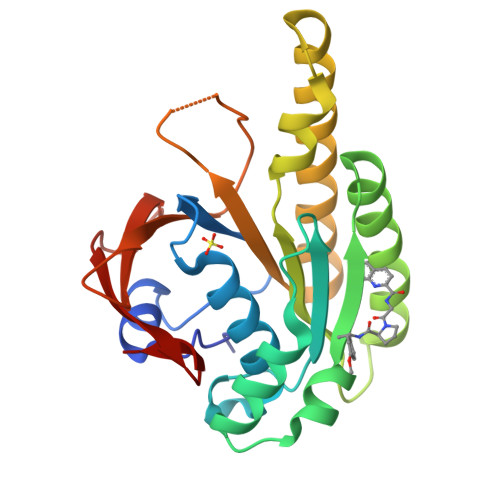
A small-molecule inhibitor of the BRCA2-RAD51 interaction modulates RAD51 assembly and potentiates DNA damage-induced cell death.
Scott, D.E., Francis-Newton, N.J., Marsh, M.E., Coyne, A.G., Fischer, G., Moschetti, T., Bayly, A.R., Sharpe, T.D., Haas, K.T., Barber, L., Valenzano, C.R., Srinivasan, R., Huggins, D.J., Lee, M., Emery, A., Hardwick, B., Ehebauer, M., Dagostin, C., Esposito, A., Pellegrini, L., Perrior, T., McKenzie, G., Blundell, T.L., Hyvonen, M., Skidmore, J., Venkitaraman, A.R., Abell, C.(2021) Cell Chem Biol 28: 835
- PubMed: 33662256
- DOI: https://doi.org/10.1016/j.chembiol.2021.02.006
- Primary Citation of Related Structures:
6TV3, 6TW3, 6TW4, 6TW9, 6XTW - PubMed Abstract:
BRCA2 controls RAD51 recombinase during homologous DNA recombination (HDR) through eight evolutionarily conserved BRC repeats, which individually engage RAD51 via the motif Phe-x-x-Ala. Using structure-guided molecular design, templated on a monomeric thermostable chimera between human RAD51 and archaeal RadA, we identify CAM833, a 529 Da orthosteric inhibitor of RAD51:BRC with a K d of 366 nM. The quinoline of CAM833 occupies a hotspot, the Phe-binding pocket on RAD51 and the methyl of the substituted α-methylbenzyl group occupies the Ala-binding pocket. In cells, CAM833 diminishes formation of damage-induced RAD51 nuclear foci; inhibits RAD51 molecular clustering, suppressing extended RAD51 filament assembly; potentiates cytotoxicity by ionizing radiation, augmenting 4N cell-cycle arrest and apoptotic cell death and works with poly-ADP ribose polymerase (PARP)1 inhibitors to suppress growth in BRCA2-wildtype cells. Thus, chemical inhibition of the protein-protein interaction between BRCA2 and RAD51 disrupts HDR and potentiates DNA damage-induced cell death, with implications for cancer therapy.
Organizational Affiliation:
Yusuf Hamied Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK.