Spatial Structure of the M2 Transmembrane Segment of the Nicotinic Acetylcholine Receptor Alpha-Subunit
Pashkov, V.S., Maslennikov, I.V., Tchikin, L.D., Efremov, R.G., Ivanov, V.T., Arseniev, A.S.(1999) FEBS Lett 457: 117
- PubMed: 10486576
- DOI: https://doi.org/10.1016/s0014-5793(99)01023-6
- Primary Citation of Related Structures:
1DXZ - PubMed Abstract:
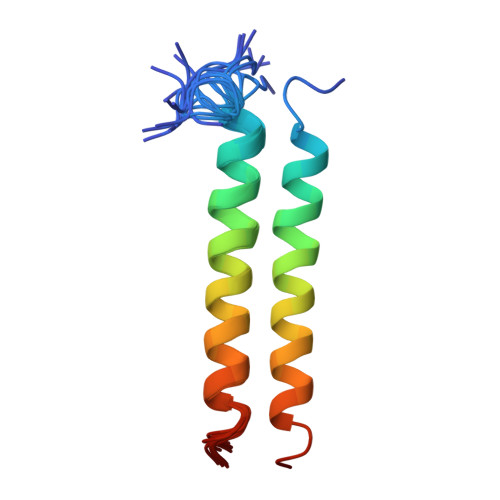
A synthetic peptide corresponding to the transmembrane segment M2 (residues 236-267) of the alpha-subunit of the nicotinic acetylcholine receptor from Torpedo californica has been studied by two dimensional 1H-NMR spectroscopy in a chloroform-methanol (1:1) mixture containing 0.1 M LiClO4. Reconstruction of the spatial structure of M2 from the NMR data resulted in an alpha-helix formed by residues 241-263. Distribution of the molecular hydrophobicity potential on the helix surface is very similar to that in five-helix bundles of proteins with a known three dimensional structure: two hydrophilic bands located on the opposite helix sides separated by strong hydrophobic zones.
Organizational Affiliation:
Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, Russia.