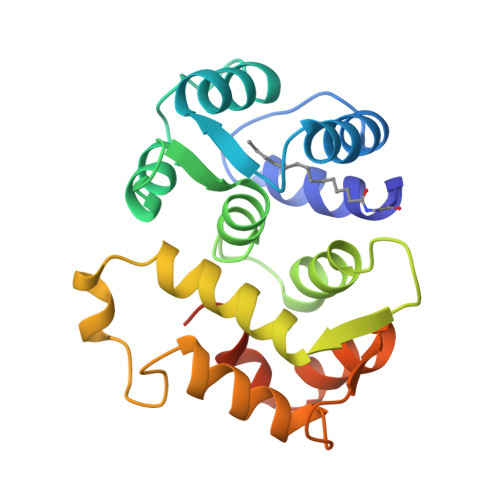
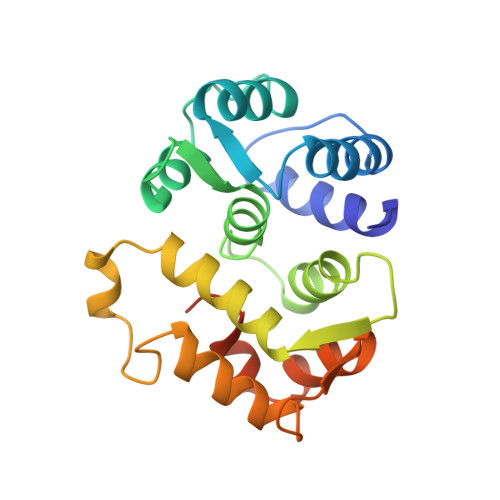
Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state.
Tanaka, T., Ames, J.B., Harvey, T.S., Stryer, L., Ikura, M.(1995) Nature 376: 444-447
- PubMed: 7630423
- DOI: https://doi.org/10.1038/376444a0
- Primary Citation of Related Structures:
1IKU - PubMed Abstract:
Recoverin, a retinal calcium-binding protein of relative molecular mass (M(r)) 23K, participates in the recovery phase of visual excitation and in adaptation to background light. The Ca(2+)-bound form of recoverin prolongs the photoresponse, probably by blocking phosphorylation of photoexcited rhodopsin. Retinal recoverin contains a covalently attached myristoyl group or related acyl group at its amino terminus and two Ca(2+)-binding sites. Ca2+ binding to myristoylated, but not unmyristoylated, recoverin induces its translocation to bilayer membranes, indicating that the myristoyl group is essential to the read-out of calcium signals (calcium-myristoyl switch). Here we present the solution structure of Ca(2+)-free, myristoylated recombinant recoverin obtained by heteronuclear multidimensional NMR spectroscopy. The myristoyl group is sequestered in a deep hydrophobic pocket formed by many aromatic and other hydrophobic residues from five flanking helices.
Organizational Affiliation:
Division of Molecular and Structural Biology, Ontario Cancer Institute, Toronto, Canada.