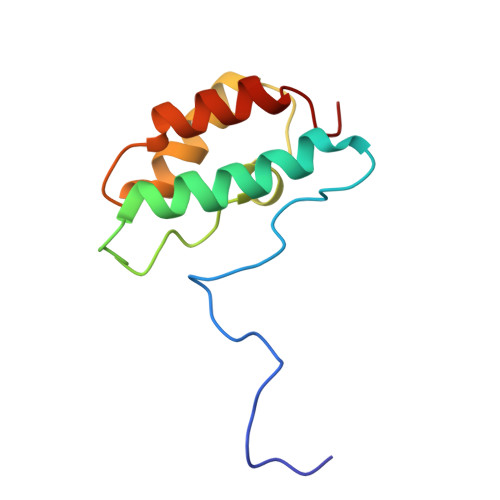
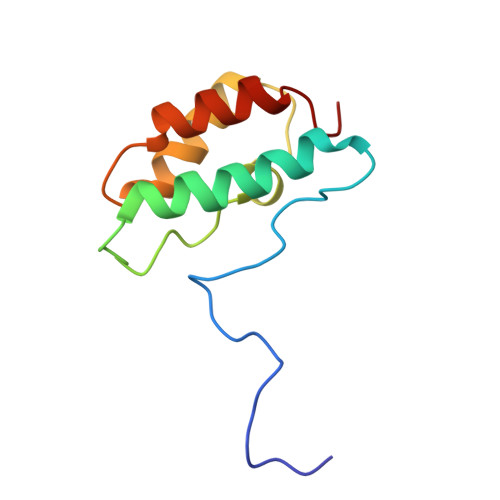
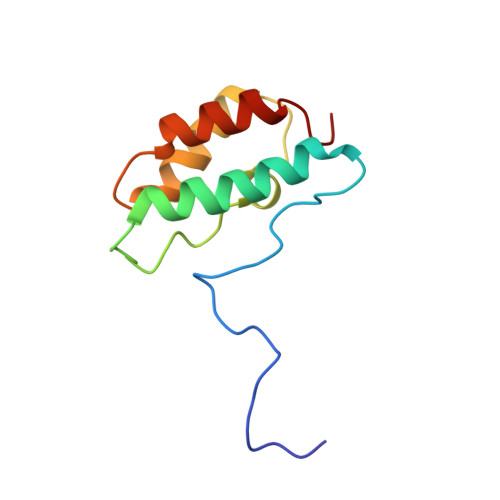
Distinctive Solution Conformation of Phosphatase Inhibitor CPI-17 Substituted with Aspartate at the Phosphorylation-site Threonine Residue
Ohki, S., Eto, M., Shimizu, M., Takada, R., Brautigan, D.L., Kainosho, M.(2003) J Mol Biology 326: 1539-1547
- PubMed: 12595264
- DOI: https://doi.org/10.1016/s0022-2836(03)00048-2
- Primary Citation of Related Structures:
1J2M, 1J2N - PubMed Abstract:
We present solution NMR structures for wild-type and mutated forms of CPI-17, a phosphoinhibitor for protein phosphatase 1. Phosphorylation of Thr38 of CPI-17 produces a >1000-fold increase in inhibitory potency for myosin phosphatase. We compared the 1H-15N heteronuclear single quantum coherence spectroscopy (HSQC) chemical shifts of wild-type CPI-17, partially phosphorylated CPI-17 and CPI-17 with Thr38 replaced with Asp to introduce a negative charge. There was a switch in the protein conformation due to either Asp substitution or phosphorylation, so we determined the solution NMR structure of the CPI-17 T38D mutant as a model for the active (phospho-) conformation. The structures reveal a molecular switch in conformation that involves the rotation of two of the four helices in the four helix bundle. Despite this conformational switch, there was little increase in the inhibitory potency with T38D. We propose that for this inhibitor, a negative charge at residue 38 is sufficient to trigger an active conformation, but a phosphoryl group is required for full inhibitory potency against protein phosphatase-1.
Organizational Affiliation:
CREST and Graduate School of Science, Tokyo Metropolitan University, 1-1 Minami-ohsawa, Hachioji, Tokyo 192-0397, Japan.