Unique Features in the C-terminal Domain Provide Caltractin with Target Specificity
Hu, H.T., Chazin, W.J.(2003) J Mol Biology 330: 473-484
- PubMed: 12842464
- DOI: https://doi.org/10.1016/s0022-2836(03)00619-3
- Primary Citation of Related Structures:
1OQP - PubMed Abstract:
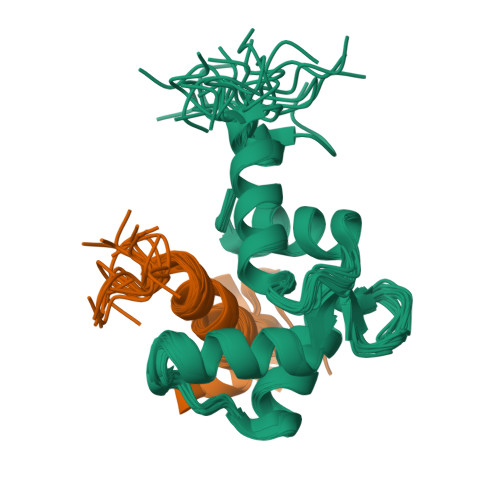
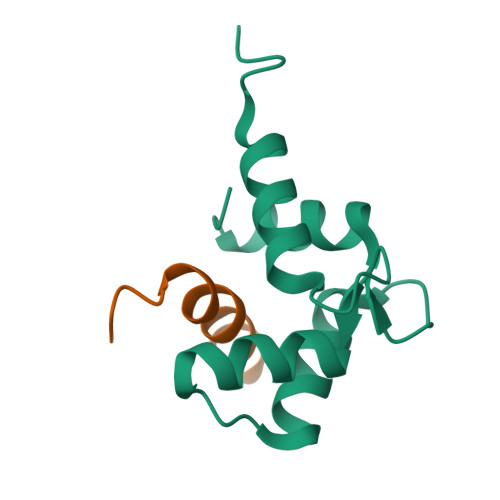
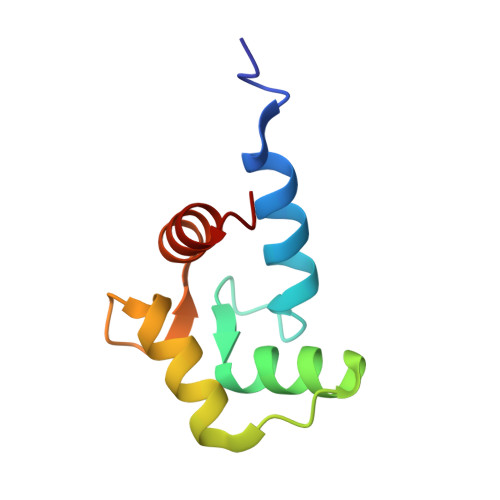
Caltractin (centrin) is a member of the calmodulin (CaM) superfamily of EF-hand calcium-binding proteins. It is an essential component of the centrosomal structures in a wide range of organisms. Caltractin and calmodulin apparently function in distinct calcium signaling pathways despite substantial sequence similarity. In an effort to understand the structural basis for such differences, the high-resolution three-dimensional solution structure of the complex between the Ca(2+)-activated C-terminal domain of Chlamydomonas reinhardtii caltractin (CRC-C) and a 19 residue peptide fragment comprising the putative cdc31p-binding region of Kar1p (K(19)) has been determined by multi-dimensional heteronuclear NMR spectroscopy. Formation of the complex is calcium-dependent and is stabilized by extensive interactions between CRC-C and three key hydrophobic anchors (Trp10, Leu13 and Leu14) in the peptide as well as favorable electrostatic interactions at the protein-peptide interface. In-depth comparisons have been made to the structure of the complex of Ca(2+)-activated calmodulin and R(20), the CaM-binding domain of smooth muscle myosin light-chain kinase. Although the overall structures of CRC and CaM domains in their respective complexes are very similar, differences in critical regions in the sequences of these proteins and their targets lead to clear differences in the complementarity of their respective binding surfaces. These subtle differences reveal the structural basis for the Ca(2+)-dependent regulation of distinct cellular signaling events by CRC and CaM.
Organizational Affiliation:
Departments of Biochemistry and Physics, Center for Structural Biology, Vanderbilt University, 5142 BIOSCI/MRBIII, Nashville, TN 37232-8725, USA.