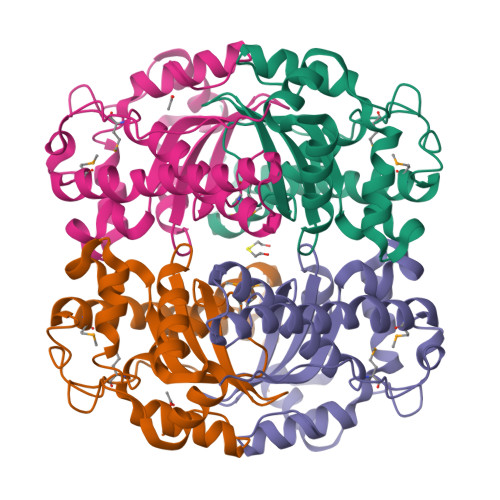
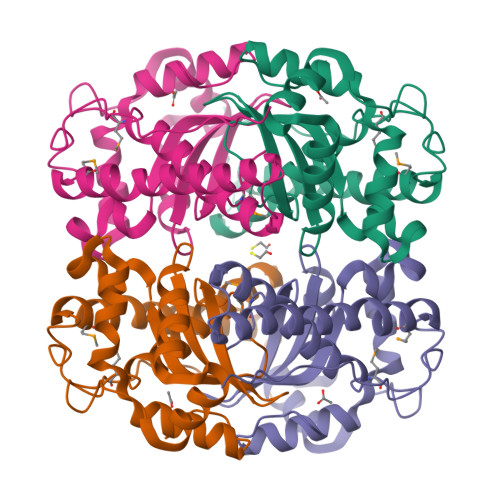
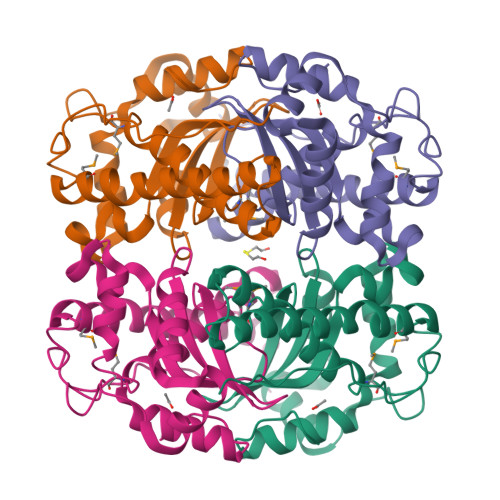
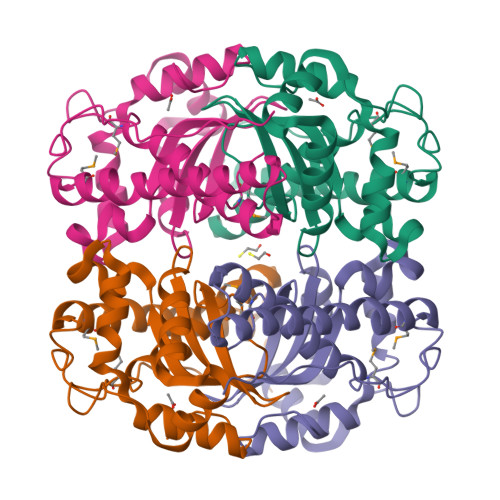
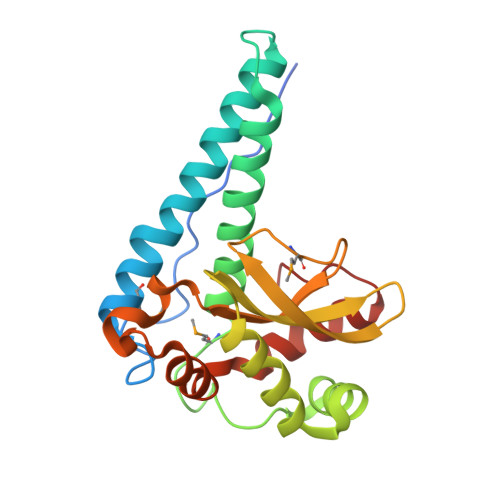
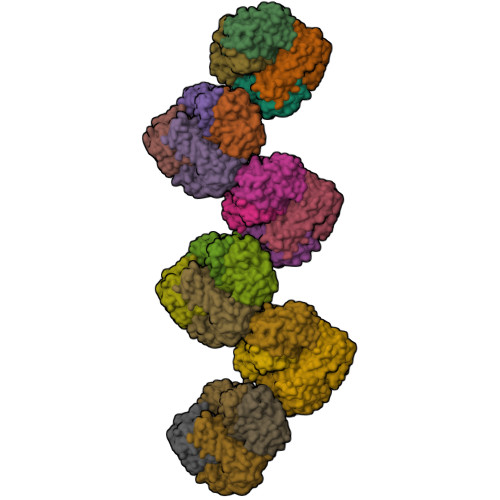Structure of superoxide dismutase from Pyrobaculum aerophilum presents a challenging case in molecular replacement with multiple molecules, pseudo-symmetry and twinning.
Lee, S., Sawaya, M.R., Eisenberg, D.(2003) Acta Crystallogr D Biol Crystallogr 59: 2191-2199
- PubMed: 14646077
- DOI: https://doi.org/10.1107/s0907444903019942
- Primary Citation of Related Structures:
1P7G - PubMed Abstract:
The crystal structure of superoxide dismutase from the hyperthermophilic crenarchaeon Pyrobaculum aerophilum was determined by molecular replacement at 1.8 A resolution. The structure determination was made especially challenging by the large number of molecules (24) in the asymmetric unit, the presence of a pseudo-crystallographic twofold operator close to a twinning operator and the inability to detect twinning by conventional means. Molecular replacement proceeded at low resolution in pseudo (apparent) space group P3(2)12 and was facilitated by examination of the self-rotation function and native Patterson map. Refinement, however, stalled at an R factor of 40% when high-resolution data were included. Expanding to the lower symmetry space group P3(2) decreased R (to 22%) and R(free) (to 26%), but not by as much as expected for the quality of data. Finally, despite the apparent lack of evidence from conventional twinning tests [i.e. plots of the second moment of I and N(Z) distributions], a twinning operator was included in the refinement, lowering R and R(free) to 16.2 and 21.7%, respectively. The early detection of twinning appears to have been masked by a deviation in the expected intensity distribution caused by the presence of non-crystallographic translational symmetry. These findings suggest the importance of testing twinning operators in cases where pseudo-translational symmetry can explain negative results from conventional twinning tests. The structure reveals a tetrameric assembly with 222 symmetry, similar to superoxide dismutase structures from other organisms. The current structural model represents the metal-free state of the enzyme.
Organizational Affiliation:
UCLA-DOE Institute for Genomics and Proteomics, Molecular Biology Institute, Howard Hughes Medical Institute, and Department of Chemistry and Biochemistry, University of California,Los Angeles, Los Angeles, CA 90095-1570, USA.