Structure-based design of novel HIV protease inhibitors: carboxamide-containing 4-hydroxycoumarins and 4-hydroxy-2-pyrones as potent nonpeptidic inhibitors.
Thaisrivongs, S., Watenpaugh, K.D., Howe, W.J., Tomich, P.K., Dolak, L.A., Chong, K.T., Tomich, C.C., Tomasselli, A.G., Turner, S.R., Strohbach, J.W., Mulichak, A.M., Janakiraman, M.N., Moon, J.B., Lynn, J.C., Horng, M.M., Hinshaw, R.R., Curry, K.A., Rothroc, D.J.(1995) J Med Chem 38: 3624-3637
- PubMed: 7658450
- DOI: https://doi.org/10.1021/jm00018a023
- Primary Citation of Related Structures:
1UPJ, 2UPJ, 3UPJ, 4UPJ - PubMed Abstract:
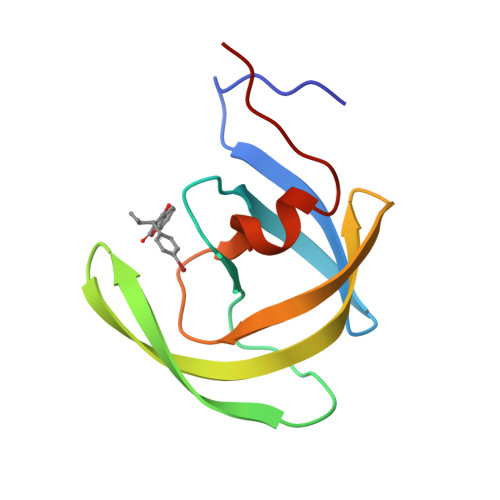
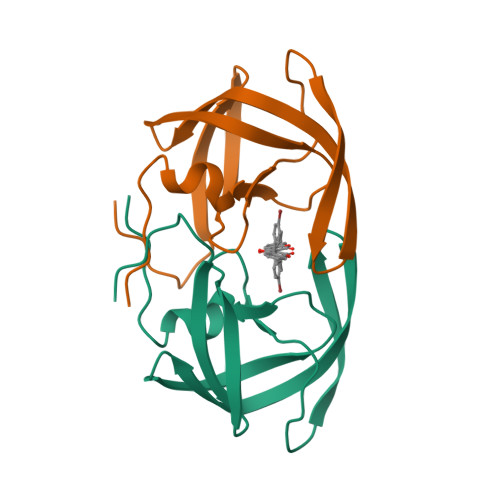
The low oral bioavailability and rapid biliary excretion of peptide-derived HIV protease inhibitors have limited their utility as potential therapeutic agents. Our broad screening program to discover nonpeptidic HIV protease inhibitors had previously identified compound II (phenprocoumon, K(i) = 1 muM) as a lead template. Crystal structures of HIV protease complexes containing the peptide-derived inhibitor I (1-(naphthoxyacetyl)-L-histidyl-5(S)-amino-6-cyclohexyl-3 (R),4(R)-dihydroxy-2(R)-isopropylhexanoyl-L-isoleucine N-(2-pyridylmethyl)amide) and nonpeptidic inhibitors, such as phenprocoumon (compound II), provided a rational basis for the structure-based design of more active analogues. This investigation reports on the important finding of a carboxamide functionally appropriately added to the 4-hydroxycoumarin and the 4-hydroxy-2-pyrone templates which resulted in a new promising series of nonpeptidic HIV protease inhibitors with improved enzyme-binding affinity. The most active diastereomer of the carboxamide-containing compound XXIV inhibited HIV-1 protease with a K(i) value of 0.0014 muM. This research provides a new design direction for the discovery of more potent HIV protease inhibitors as potential therapeutic agents for the treatment of HIV infection.
Organizational Affiliation:
Upjohn Laboratories, Kalamazoo, Michigan 49001, USA.