The structure of phosphate-bound Escherichia coli adenylosuccinate lyase identifies His171 as a catalytic acid.
Kozlov, G., Nguyen, L., Pearsall, J., Gehring, K.(2009) Acta Crystallogr Sect F Struct Biol Cryst Commun 65: 857-861
- PubMed: 19724117
- DOI: https://doi.org/10.1107/S1744309109029674
- Primary Citation of Related Structures:
3GZH - PubMed Abstract:
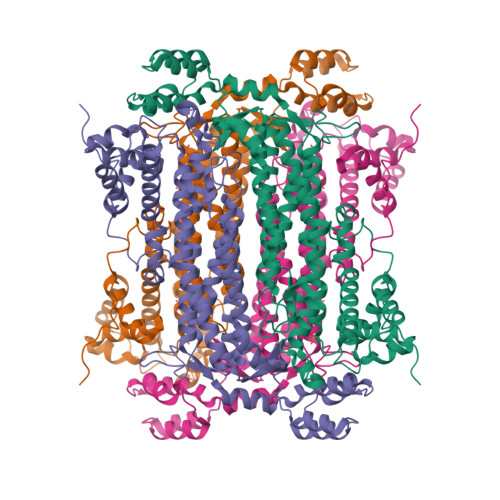
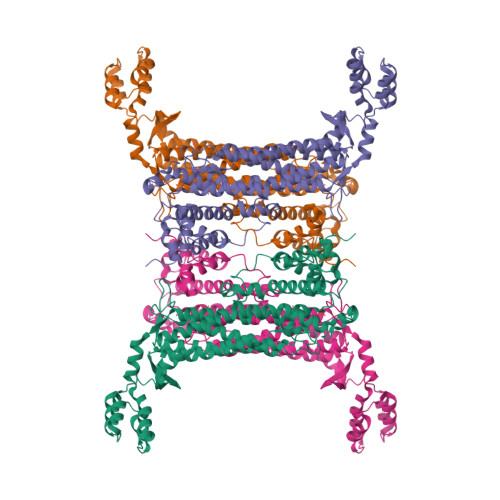
Adenylosuccinate lyase (ASL) is an enzyme from the purine-biosynthetic pathway that catalyzes the cleavage of 5-aminoimidazole-4-(N-succinylcarboxamide) ribonucleotide (SAICAR) to 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) and fumarate. ASL is also responsible for the conversion of succinyladenosine monophosphate (SAMP) to adenosine monophosphate (AMP) and fumarate. Here, the crystal structure of adenylosuccinate lyase from Escherichia coli was determined to 1.9 A resolution. The enzyme adopts a substrate-bound conformation as a result of the presence of two phosphate ions bound in the active site. Comparison with previously solved structures of the apoenzyme and an SAMP-bound H171A mutant reveals a conformational change at His171 associated with substrate binding and confirms the role of this residue as a catalytic acid.
Organizational Affiliation:
Department of Biochemistry, McGill University, Montreal, Quebec, Canada.