Structural basis for the recognition-evasion arms race between Tomato mosaic virus and the resistance gene Tm-1
Ishibashi, K., Kezuka, Y., Kobayashi, C., Kato, M., Inoue, T., Nonaka, T., Ishikawa, M., Matsumura, H., Katoh, E.(2014) Proc Natl Acad Sci U S A 111: E3486-E3495
- PubMed: 25092327
- DOI: https://doi.org/10.1073/pnas.1407888111
- Primary Citation of Related Structures:
3WRV, 3WRW, 3WRX, 3WRY - PubMed Abstract:
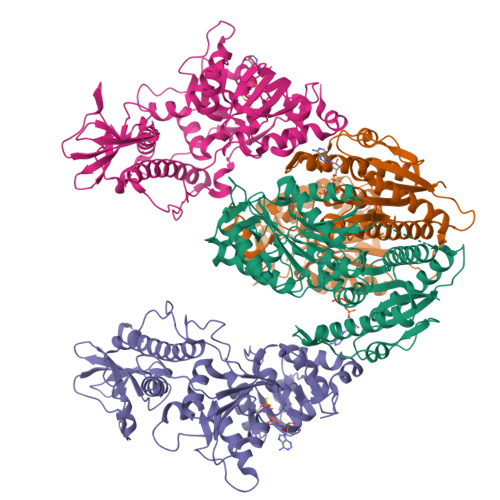
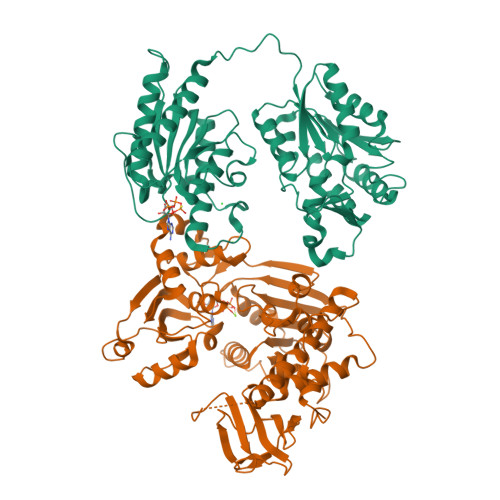
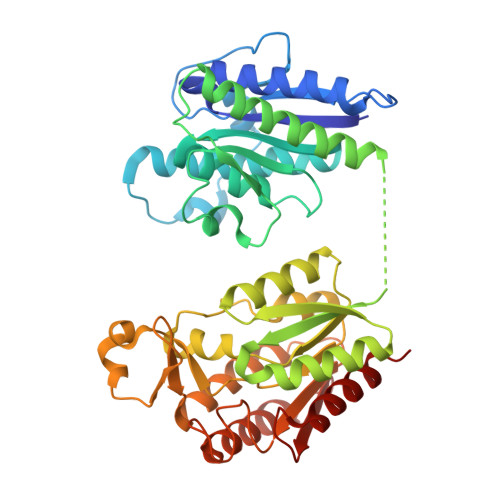
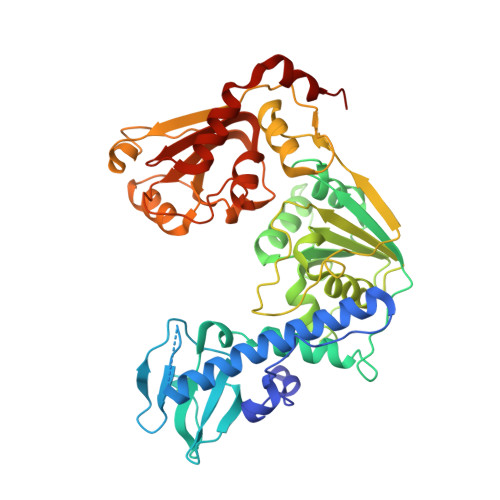
The tomato mosaic virus (ToMV) resistance gene Tm-1 encodes a protein that shows no sequence homology to functionally characterized proteins. Tm-1 binds ToMV replication proteins and thereby inhibits replication complex formation. ToMV mutants that overcome this resistance have amino acid substitutions in the helicase domain of the replication proteins (ToMV-Hel). A small region of Tm-1 in the genome of the wild tomato Solanum habrochaites has been under positive selection during its antagonistic coevolution with ToMV. Here we report crystal structures for the N-terminal inhibitory domains of Tm-1 and a natural Tm-1 variant with an I91-to-T substitution that has a greater ability to inhibit ToMV RNA replication and their complexes with ToMV-Hel. Each complex contains a Tm-1 dimer and two ToMV-Hel monomers with the interfaces between Tm-1 and ToMV-Hel bridged by ATP. Residues in ToMV-Hel and Tm-1 involved in antagonistic coevolution are found at the interface. The structural differences between ToMV-Hel in its free form and in complex with Tm-1 suggest that Tm-1 affects nucleoside triphosphatase activity of ToMV-Hel, and this effect was confirmed experimentally. Molecular dynamics simulations of complexes formed by Tm-1 with ToMV-Hel variants showed how the amino acid changes in ToMV-Hel impair the interaction with Tm-1 to overcome the resistance. With these findings, together with the biochemical properties of the interactions between ToMV-Hel and Tm-1 variants and effects of the mutations in the polymorphic residues of Tm-1, an atomic view of a step-by-step coevolutionary arms race between a plant resistance protein and a viral protein emerges.
Organizational Affiliation:
Plant-Microbe Interactions Research Unit and.