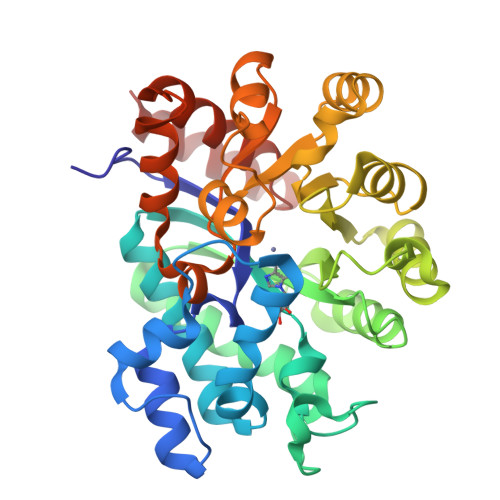
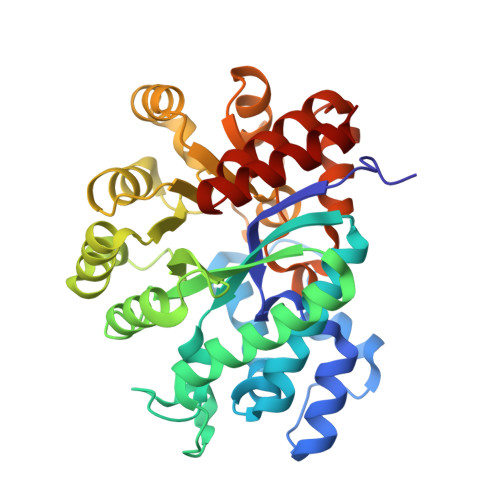
A pre-transition-state mimic of an enzyme: X-ray structure of adenosine deaminase with bound 1-deazaadenosine and zinc-activated water.
Wilson, D.K., Quiocho, F.A.(1993) Biochemistry 32: 1689-1694
- PubMed: 8439534
- DOI: https://doi.org/10.1021/bi00058a001
- Primary Citation of Related Structures:
1ADD - PubMed Abstract:
The refined 2.4-A structure of adenosine deaminase, recently discovered to be a zinc metalloenzyme [Wilson, D. K., Rudolph, F. B., & Quiocho, F. A. (1991) Science 252, 1278-1284], complexed with the ground-state analog 1-deazaadenosine shows the mode of binding of the analog and, unexpectedly, a zinc-activated water (hydroxide). This structure of a pre-transition-state mimic, combined with that previously determined for the complex with 6(R)-hydroxy-1,6-dihydropurine ribonucleoside, a nearly ideal transition-state analog, sheds new understanding of the precise stereospecificity and hydrolytic catalysis of an important and well-characterized member of a large group of zinc metalloenzymes. As both of these excellent mimics were generated in the active site, they demonstrate a powerful means of dissecting the course of an enzymatic reaction by direct crystallographic analysis.
Organizational Affiliation:
Howard Hughes Medical Institute, Baylor College of Medicine, Houston, Texas 77030.