Allergic Cross-Reactivity Made Visible: The Solution Structure of the Major Cherry Allergen Pru Av 1
Neudecker, P., Schweimer, K., Nerkamp, J., Scheurer, S., Vieths, S., Sticht, H., Roesch, P.(2001) J Biological Chem 276: 22756
- PubMed: 11287426
- DOI: https://doi.org/10.1074/jbc.M101657200
- Primary Citation of Related Structures:
1E09 - PubMed Abstract:
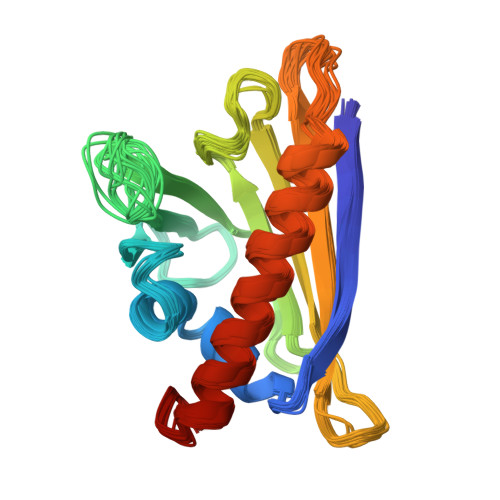
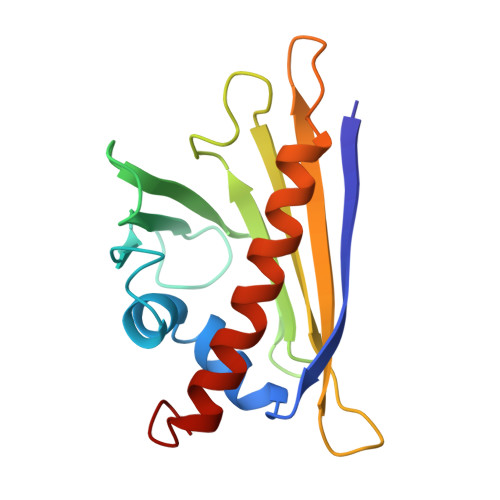
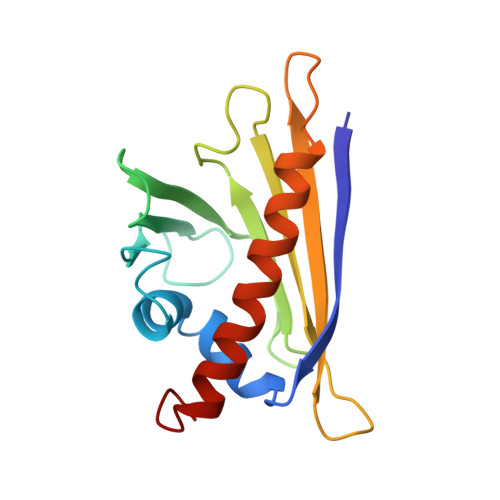
Birch pollinosis is often accompanied by hypersensitivity to fruit as a consequence of the cross-reaction of pollen allergen-specific IgE antibodies with homologous food proteins. To provide a basis for examining the cross-reactivity on a structural level, we used heteronuclear multidimensional NMR spectroscopy to determine the high-resolution three-dimensional structure of the major cherry allergen, Pru av 1, in solution. Based on a detailed comparison of the virtually identical structures of Pru av 1 and Bet v 1, the major birch pollen allergen, we propose an explanation for a significant aspect of the observed cross-reactivity pattern among the family of allergens under consideration. The large hydrophobic cavity expected to be important for the still unknown physiological function of Bet v 1 is conserved in Pru av 1. Structural homology to a domain of human MLN64 associated with cholesterol transport suggests phytosteroids as putative ligands for Pru av 1. NMR spectroscopy provides experimental evidence that Pru av 1 interacts with phytosteroids, and molecular modeling shows that the hydrophobic cavity is large enough to accommodate two such molecules.
Organizational Affiliation:
Lehrstuhl für Biopolymere, Universität Bayreuth, D-95440 Bayreuth, Germany.