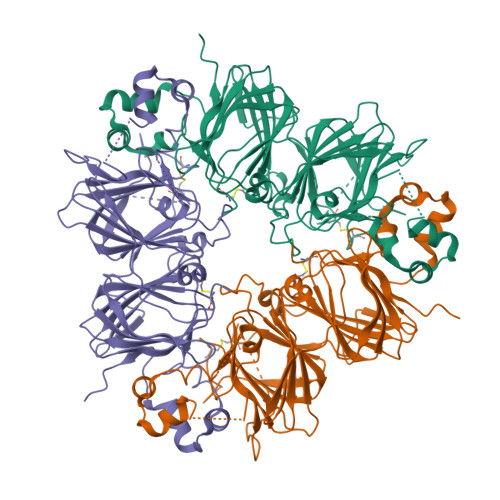
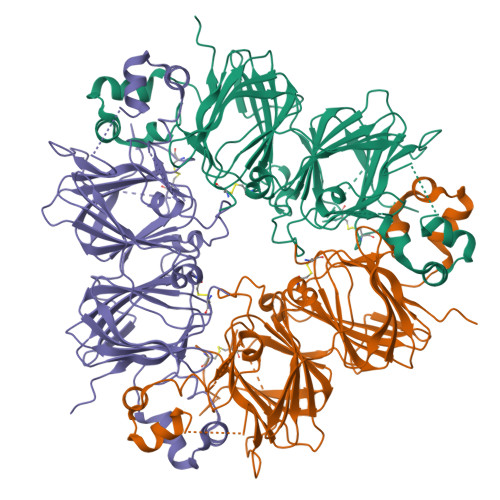
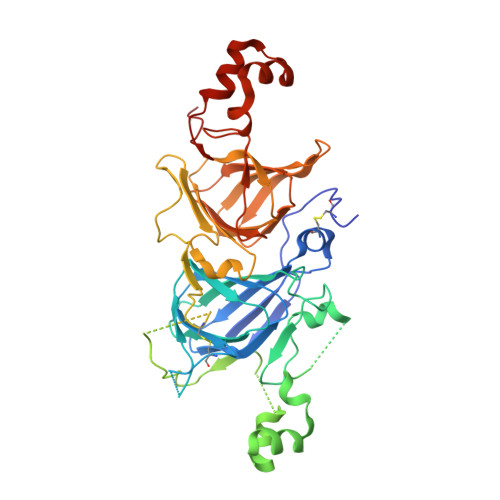
Crystal structure of soybean proglycinin A1aB1b homotrimer.
Adachi, M., Takenaka, Y., Gidamis, A.B., Mikami, B., Utsumi, S.(2001) J Mol Biology 305: 291-305
- PubMed: 11124907
- DOI: https://doi.org/10.1006/jmbi.2000.4310
- Primary Citation of Related Structures:
1FXZ - PubMed Abstract:
Soybean glycinin is a member of the 11 S globulin family. The crystal structure of proglycinin was determined by X-ray crystallography at 2.8 A resolution with an R-factor of 0.199 and a free R-factor of 0.250. A trimer molecule was found in an asymmetric unit of crystals. The trimer model contains three A1aB1b subunits and comprises 1128 amino acid residues and 34 water molecules. The constituent protomers of the homo-trimeric protein are arranged around a 3-fold symmetry axis with dimensions of 95 Ax95 Ax40 A. The protomer model is composed of five fragments which correspond roughly to conserved regions based on the sequence alignment of various 11 S globulins. The core of the protomer consists of two jelly-roll beta-barrels and two extended helix domains. This structure of proglycinin is similar to those of canavalin and phaseolin belonging to the 7 S globulin family, strongly supporting the hypothesis that both 7 S and 11 S globulins are derived from a common ancestor. The inter and intra-chain disulfide bonds conserved in the 11 S globulin family are clearly observed. It is found that the face with the inter-chain disulfide bond (IE face) contains more hydrophobic residues than that with the intra-chain disulfide bond. This suggests that a mature hexamer is formed by the interaction between the IE faces after processing.
Organizational Affiliation:
Research Institute for Food Science, Kyoto University, Uji, Kyoto, 611-0011, Japan.