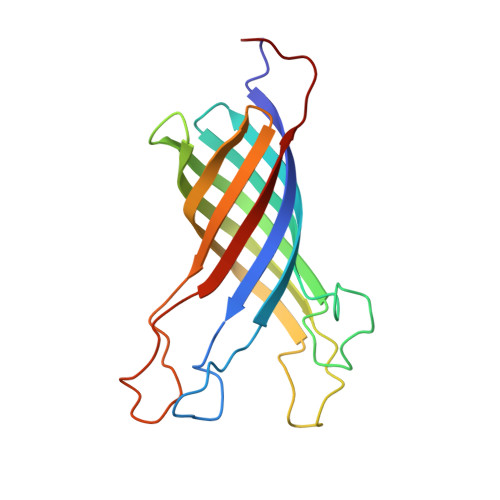
Structure of outer membrane protein A transmembrane domain by NMR spectroscopy
Arora, A., Abildgaard, F., Bushweller, J.H., Tamm, L.K.(2001) Nat Struct Biol 8: 334-338
- PubMed: 11276254
- DOI: https://doi.org/10.1038/86214
- Primary Citation of Related Structures:
1G90 - PubMed Abstract:
We have determined the three-dimensional fold of the 19 kDa (177 residues) transmembrane domain of the outer membrane protein A of Escherichia coli in dodecylphosphocholine (DPC) micelles in solution using heteronuclear NMR. The structure consists of an eight-stranded beta-barrel connected by tight turns on the periplasmic side and larger mobile loops on the extracellular side. The solution structure of the barrel in DPC micelles is similar to that in n-octyltetraoxyethylene (C(8)E(4)) micelles determined by X-ray diffraction. Moreover, data from NMR dynamic experiments reveal a gradient of conformational flexibility in the structure that may contribute to the membrane channel function of this protein.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, Virginia 22908-0736, USA.