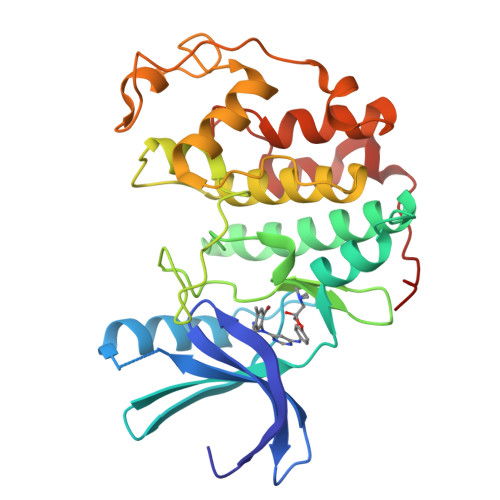
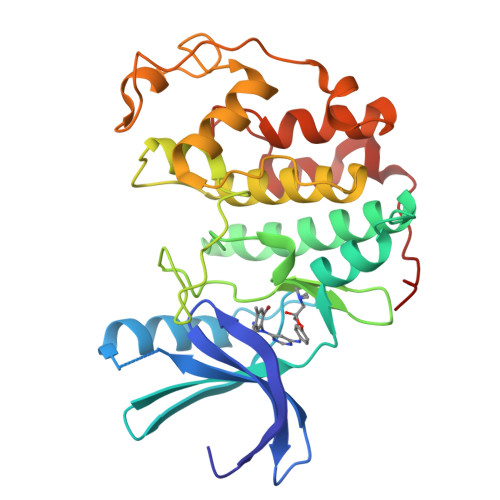
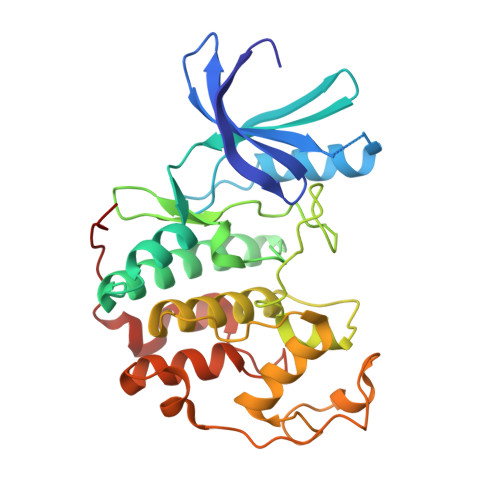
Cyclin-Dependent Kinase 4 Inhibitors as a Treatment for Cancer. Part 1: Identification and Optimisation of Substituted 4,6-Bis Anilino Pyrimidines
Beattie, J.F., Breault, G.A., Ellston, R.P.A., Green, S., Jewsbury, P.J., Midgley, C.J., Naven, R.T., Minshull, C.A., Pauptit, R.A., Tucker, J.A., Pease, J.E.(2003) Bioorg Med Chem Lett 13: 2955
- PubMed: 12941311
- DOI: https://doi.org/10.1016/s0960-894x(03)00202-6
- Primary Citation of Related Structures:
1H00, 1H01, 1H07, 1H08, 1V1K - PubMed Abstract:
Using a high-throughput screening campaign, we identified the 4,6-bis anilino pyrimidines as inhibitors of the cyclin-dependent kinase, CDK4. Herein we describe the further chemical modification and use of X-ray crystallography to develop potent and selective in vitro inhibitors of CDK4.
Organizational Affiliation:
AstraZeneca, Alderley Park, Macclesfield, Cheshire SK10 4TG, UK.