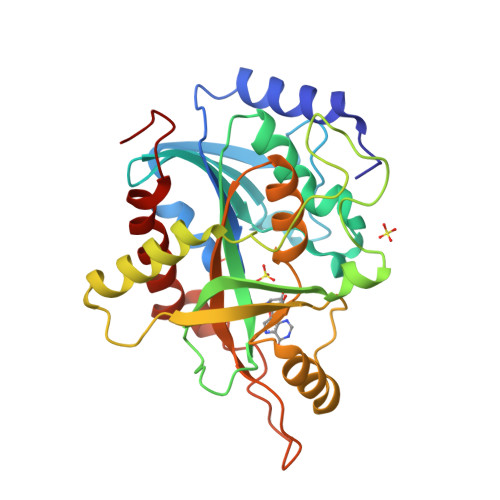
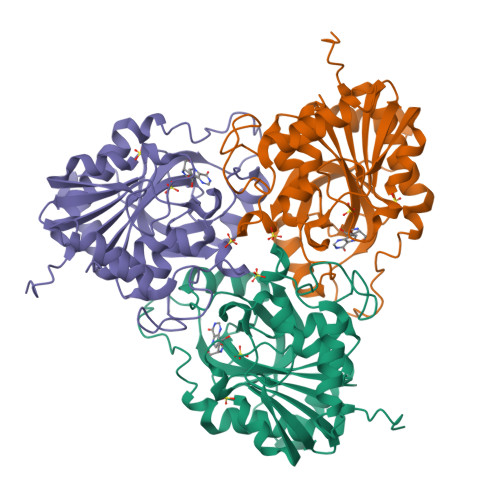
Structural basis for inhibition of human PNP by immucillin-H
De Azevedo Jr., W.F., Canduri, F., Dos Santos, D.M., Pereira, J.H., Dias, M.V.B., Silva, R.G., Mendes, M.A., Palma, M.S., Basso, L.A., Santos, D.S.(2003) Biochem Biophys Res Commun 309: 917-922
- PubMed: 13679061
- DOI: https://doi.org/10.1016/j.bbrc.2003.08.094
- Primary Citation of Related Structures:
1PF7 - PubMed Abstract:
Purine nucleoside phosphorylase (PNP) catalyzes the phosphorolysis of the N-ribosidic bonds of purine nucleosides and deoxynucleosides. PNP is a target for inhibitor development aiming at T-cell immune response modulation. This work reports on the crystallographic study of the complex of human PNP-immucillin-H (HsPNP-ImmH) solved at 2.6A resolution using synchrotron radiation. Immucillin-H (ImmH) inhibits the growth of malignant T-cell lines in the presence of deoxyguanosine without affecting non-T-cell tumor lines. ImmH inhibits activated normal human T cells after antigenic stimulation in vitro. These biological effects of ImmH suggest that this agent may have utility in the treatment of certain human diseases characterized by abnormal T-cell growth or activation. This is the first structural report of human PNP complexed with immucillin-H. The comparison of the complex HsPNP-ImmH with recent crystallographic structures of human PNP explains the high specificity of immucillin-H for human PNP.
Organizational Affiliation:
Departamento de Física, UNESP, São José do Rio Preto, SP 15054-000, Brazil. walterfa@df.ibilce.unesp.br