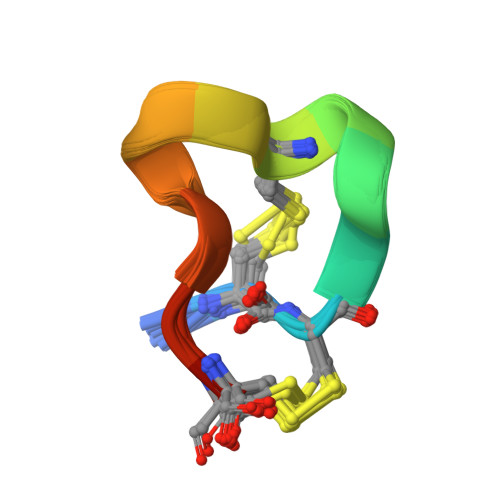
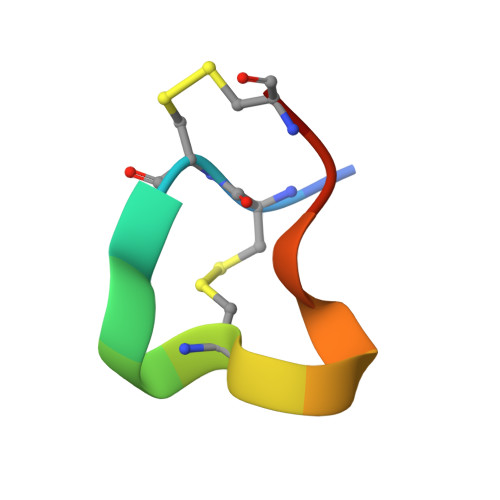
Solution Structure of Alpha-Conotoxin Si
Benie, A.J., Whitford, D., Hargittai, B., Barany, G., Janes, R.W.(2000) FEBS Lett 476: 287
- PubMed: 10913630
- DOI: https://doi.org/10.1016/s0014-5793(00)01724-5
- Primary Citation of Related Structures:
1QMW - PubMed Abstract:
The nuclear magnetic resonance solution structure of alpha-conotoxin SI has been determined at pH 4.2. The 36 lowest energy structures show that alpha-conotoxin SI exists in a single major solution conformation and is stabilized by six hydrogen bonds. Comparisons are made between the SI solution structure and the solution and crystal structures of alpha-conotoxin GI. Surprisingly, a high degree of similarity between the backbone conformations of the GI crystal and the SI solution structures is seen in the region of lowest sequence homology, namely residues Gly-8 to Ser-12. This similarity is more surprising when considering that in SI a proline replaces the Arg-9 found in GI. The correspondence in conformation in this region provides the definitive evidence that it is the loss of the arginine basic charge at residue 9 which determines the differences in toxicity between GI and SI, rather than any changes in conformation induced by the cyclic proline residue.
Organizational Affiliation:
Molecular and Cellular Biology, St. Bartholomew's and the Royal London School of Medicine and Dentistry, University of London, UK.