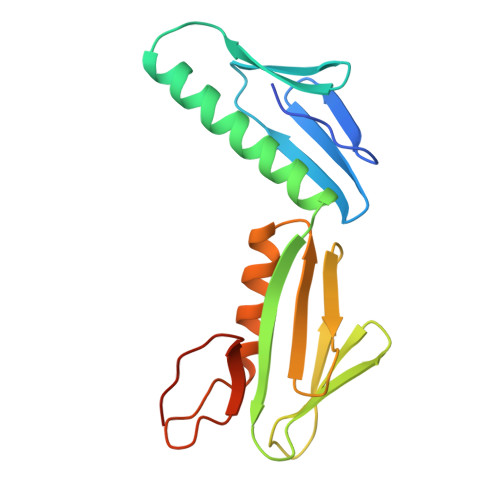
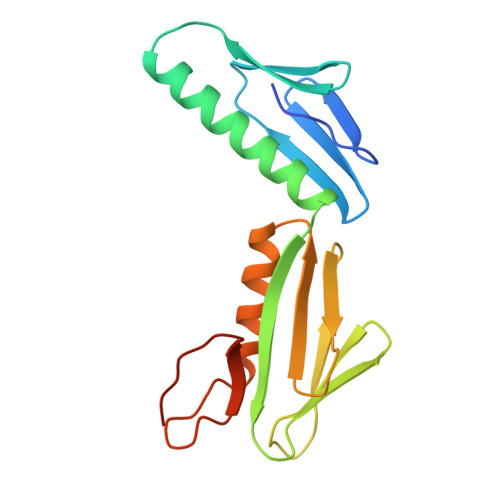
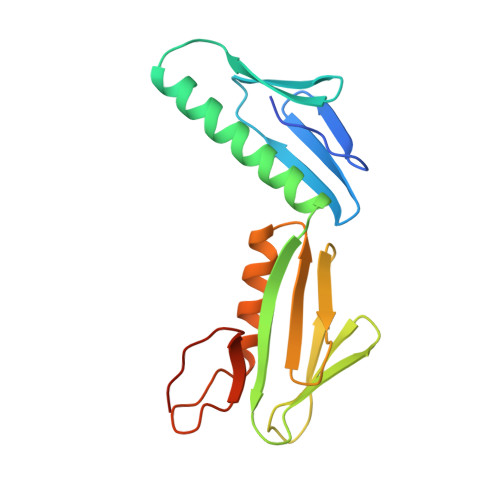
Ribosomal protein L6: structural evidence of gene duplication from a primitive RNA binding protein.
Golden, B.L., Ramakrishnan, V., White, S.W.(1993) EMBO J 12: 4901-4908
- PubMed: 8262035
- DOI: https://doi.org/10.1002/j.1460-2075.1993.tb06184.x
- Primary Citation of Related Structures:
1RL6 - PubMed Abstract:
In all cells, protein synthesis is coordinated by the ribosome, a large ribonucleoprotein particle that is composed of > 50 distinct protein molecules and several large RNA molecules. Here we present the crystal structure of ribosomal protein L6 from the thermophilic bacterium Bacillus stearothermophilus solved at 2.6 A resolution. L6 contains two domains with almost identical folds, implying that it was created by an ancient gene duplication event. The surface of the molecule displays several likely sites of interaction with other components of the ribosome. The RNA binding sites appear to be localized in the C-terminal domain whereas the N-terminal domain contains the potential sites for protein-protein interactions. The domain structure is homologous with several other ribosomal proteins and to a large family of eukaryotic RNA binding proteins.
Organizational Affiliation:
Department of Microbiology, Duke University Medical Center, Durham, NC 27710.