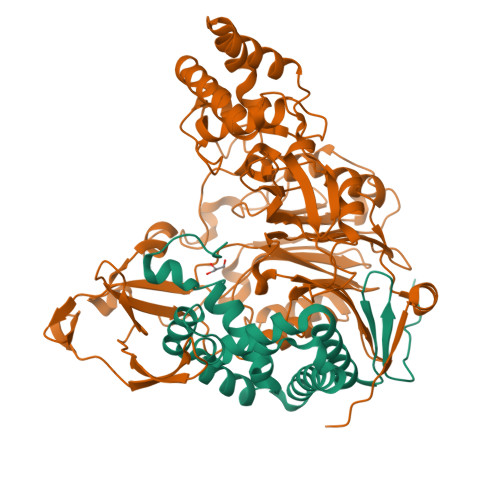
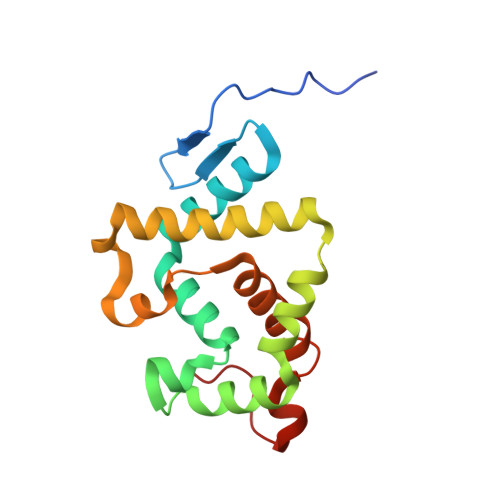
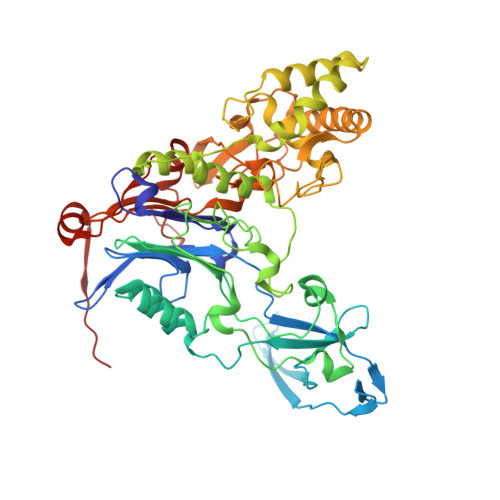
Insight into autoproteolytic activation from the structure of cephalosporin acylase: a protein with two proteolytic chemistries.
Kim, J.K., Yang, I.S., Shin, H.J., Cho, K.J., Ryu, E.K., Kim, S.H., Park, S.S., Kim, K.H.(2006) Proc Natl Acad Sci U S A 103: 1732-1737
- PubMed: 16446446
- DOI: https://doi.org/10.1073/pnas.0507862103
- Primary Citation of Related Structures:
2ADV, 2AE3, 2AE4, 2AE5 - PubMed Abstract:
Cephalosporin acylase (CA), a member of the N-terminal nucleophile hydrolase family, is activated through sequential primary and secondary autoproteolytic reactions with the release of a pro segment. We have determined crystal structures of four CA mutants. Two mutants are trapped after the primary cleavage, and the other two undergo secondary cleavage slowly. These structures provide a look at pro-segment conformation during activation in N-terminal nucleophile hydrolases. The highly strained helical pro segment of precursor is transformed into a relaxed loop in the intermediates, suggesting that the relaxation of structural constraints drives the primary cleavage reaction. The secondary autoproteolytic step has been proposed to be intermolecular. However, our analysis provides evidence that CA is processed in two sequential steps of intramolecular autoproteolysis involving two distinct residues in the active site, the first a serine and the second a glutamate.
Organizational Affiliation:
Department of Life Sciences and Biotechnology, School of Life Sciences and Biotechnology, Korea University, Seoul 136-701, Korea.