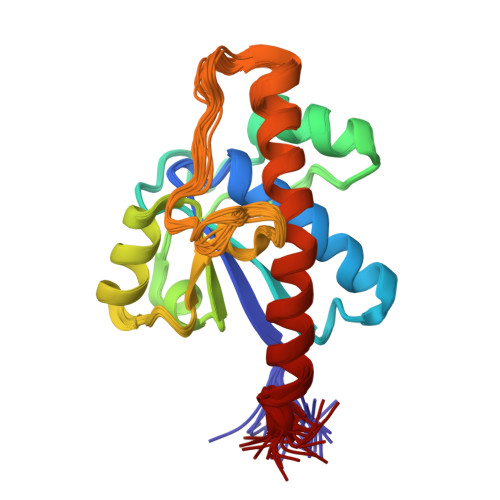
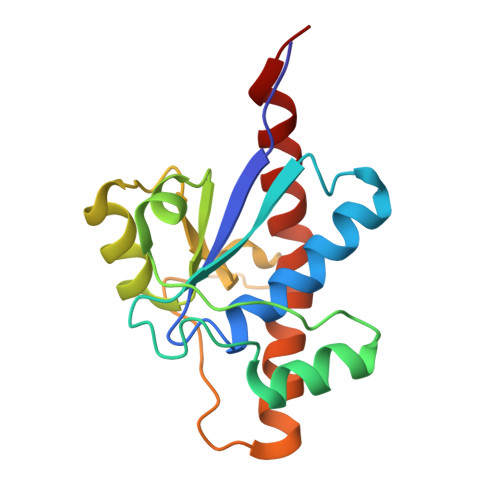
The Apo-structure of the Low Molecular Weight Protein-tyrosine Phosphatase A (MptpA) from Mycobacterium tuberculosis Allows for Better Target-specific Drug Development.
Stehle, T., Sreeramulu, S., Lohr, F., Richter, C., Saxena, K., Jonker, H.R., Schwalbe, H.(2012) J Biological Chem 287: 34569-34582
- PubMed: 22888002
- DOI: https://doi.org/10.1074/jbc.M112.399261
- Primary Citation of Related Structures:
2LUO - PubMed Abstract:
Protein-tyrosine phosphatases (PTPs) and protein-tyrosine kinases co-regulate cellular processes. In pathogenic bacteria, they are frequently exploited to act as key virulence factors for human diseases. Mycobacterium tuberculosis, the causative organism of tuberculosis, secretes a low molecular weight PTP (LMW-PTP), MptpA, which is required for its survival upon infection of host macrophages. Although there is otherwise no sequence similarity of LMW-PTPs to other classes of PTPs, the phosphate binding loop (P-loop) CX(5)R and the loop containing a critical aspartic acid residue (D-loop), required for the catalytic activity, are well conserved. In most high molecular weight PTPs, ligand binding to the P-loop triggers a large conformational reorientation of the D-loop, in which it moves ∼10 Å, from an "open" to a "closed" conformation. Until now, there have been no ligand-free structures of LMW-PTPs described, and hence the dynamics of the D-loop have remained largely unknown for these PTPs. Here, we present a high resolution solution NMR structure of the free form of the MptpA LMW-PTP. In the absence of ligand and phosphate ions, the D-loop adopts an open conformation. Furthermore, we characterized the binding site of phosphate, a competitive inhibitor of LMW-PTPs, on MptpA and elucidated the involvement of both the P- and D-loop in phosphate binding. Notably, in LMW-PTPs, the phosphorylation status of two well conserved tyrosine residues, typically located in the D-loop, regulates the enzyme activity. PtkA, the kinase complementary to MptpA, phosphorylates these two tyrosine residues in MptpA. We characterized the MptpA-PtkA interaction by NMR spectroscopy to show that both the P- and D-loop form part of the binding interface.
Organizational Affiliation:
Institute for Organic Chemistry and Chemical Biology, ohann Wolfgang Goethe University, Max-von-Laue-Strasse 7, D-60438 Frankfurt am Main, Germany.