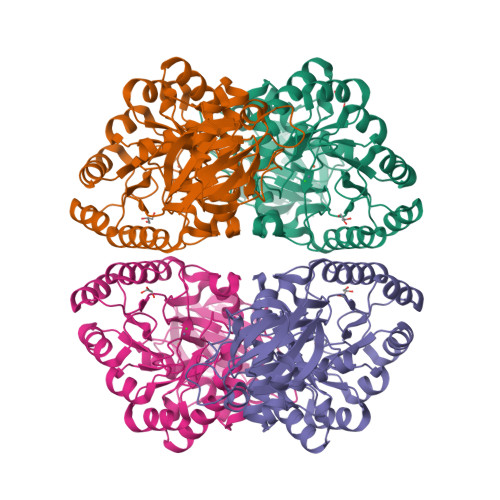
The Crystal Structure of Enamidase: A Bifunctional Enzyme of the Nicotinate Catabolism.
Kress, D., Alhapel, A., Pierik, A.J., Essen, L.-O.(2008) J Mol Biology 384: 837
- PubMed: 18805424
- DOI: https://doi.org/10.1016/j.jmb.2008.09.002
- Primary Citation of Related Structures:
2VUN - PubMed Abstract:
The hydrolysis of 1,4,5,6-tetrahydro-6-oxonicotinate to 2-formylglutarate is a central step in the catabolism of nicotinate in several Clostridia and Proteobacteria. This reaction is catalyzed by the novel enzyme enamidase, a new member of the amidohydrolase superfamily as indicated by its unique reaction, sequence relationship, and the stoichiometric binding of iron and zinc. A hallmark of enamidase is its capability to catalyze a two-step reaction: the initial decyclization of 1,4,5,6-tetrahydro-6-oxonicotinate leading to 2-(enamine)glutarate followed by an additional hydrolysis step yielding (S)-2-formylglutarate. Here, we present the crystal structure of enamidase from Eubacterium barkeri at 1.9 A resolution, providing a structural basis for catalysis and suggesting a mechanism for its exceptional activity and enantioselectivity. The enzyme forms a 222-symmetric tetramer built up by a dimer of dimers. Each enamidase monomer consists of a composite beta-sandwich domain and an (alpha/beta)(8)-TIM-barrel domain harboring the active site. With its catalytic binuclear metal center comprising both zinc and iron ions, enamidase represents a special case of subtype II amidohydrolases.
Organizational Affiliation:
Philipps-Universität Marburg, Fachbereich Chemie, Marburg, Germany.