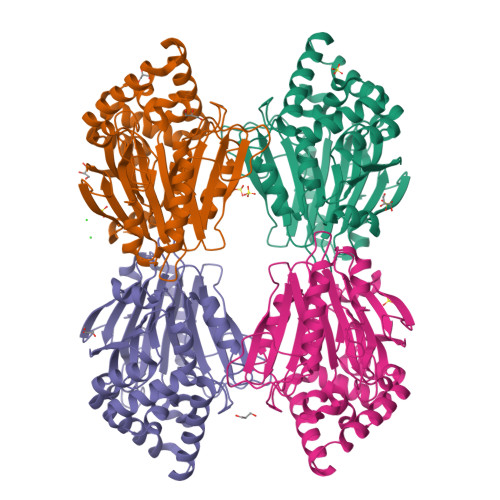
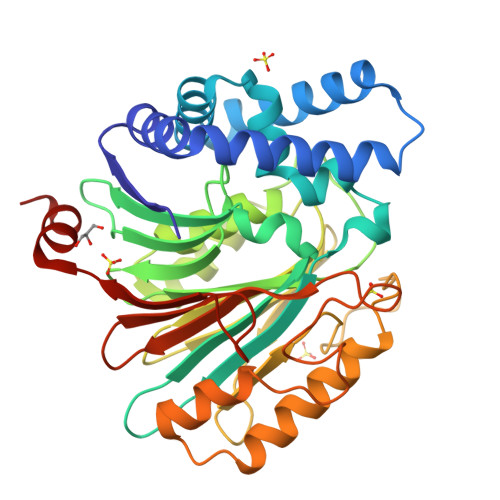
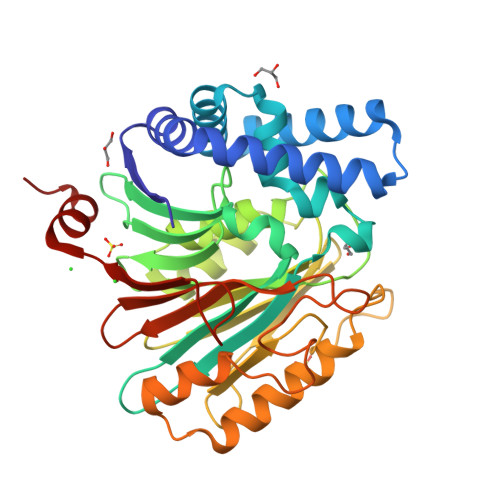
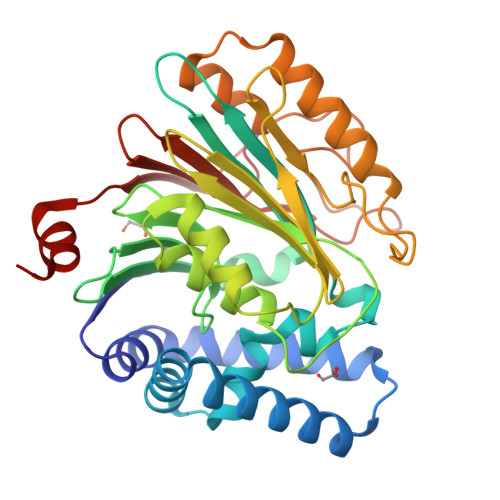
Structures of an Isopenicillin N Converting Ntn-Hydrolase Reveal Different Catalytic Roles for the Active Site Residues of Precursor and Mature Enzyme.
Bokhove, M., Yoshida, H., Hensgens, C.M.H., Van Der Laan, J.M., Sutherland, J.D., Dijkstra, B.W.(2010) Structure 18: 301
- PubMed: 20223213
- DOI: https://doi.org/10.1016/j.str.2010.01.005
- Primary Citation of Related Structures:
2X1C, 2X1D, 2X1E - PubMed Abstract:
Penicillium chrysogenum Acyl coenzyme A:isopenicillin N acyltransferase (AT) performs the last step in the biosynthesis of hydrophobic penicillins, exchanging the hydrophilic side chain of a precursor for various hydrophobic side chains. Like other N-terminal nucleophile hydrolases AT is produced as an inactive precursor that matures upon posttranslational cleavage. The structure of a Cys103Ala precursor mutant shows that maturation is autoproteolytic, initiated by Cys103 cleaving its preceding peptide bond. The crystal structure of the mature enzyme shows that after autoproteolysis residues 92-102 fold outwards, exposing a buried pocket. This pocket is structurally and chemically flexible and can accommodate substrates of different size and polarity. Modeling of a substrate-bound state indicates the residues important for catalysis. Comparison of the proposed autoproteolytic and substrate hydrolysis mechanisms shows that in both events the same catalytic residues are used, but that they perform different roles in catalysis.
Organizational Affiliation:
Laboratory of Biophysical Chemistry, University of Groningen, Nijenborgh 4, Groningen, Netherlands.