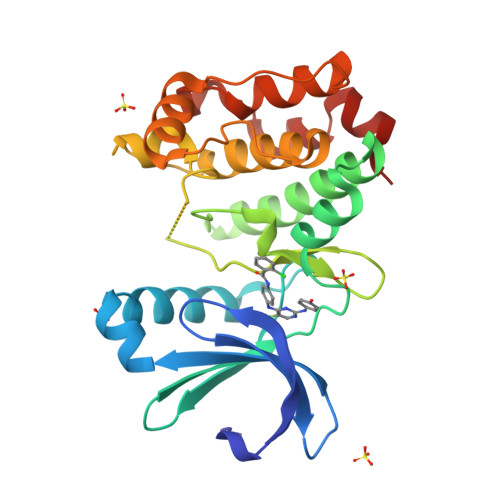
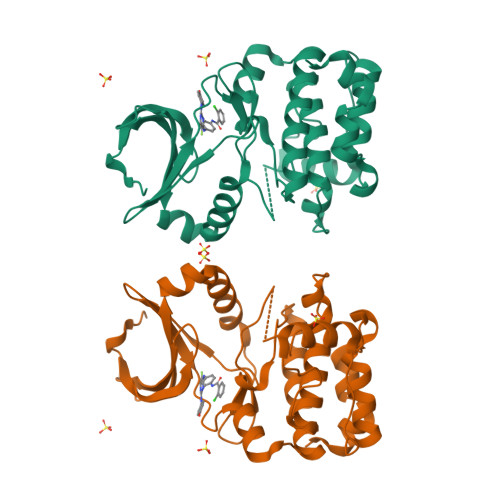
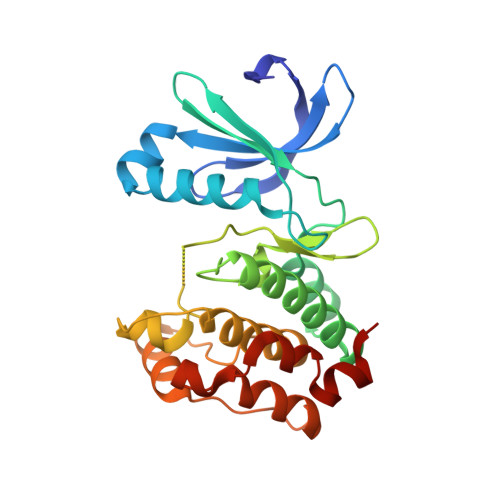
A class of 2,4-bisanilinopyrimidine Aurora A inhibitors with unusually high selectivity against Aurora B.
Aliagas-Martin, I., Burdick, D., Corson, L., Dotson, J., Drummond, J., Fields, C., Huang, O.W., Hunsaker, T., Kleinheinz, T., Krueger, E., Liang, J., Moffat, J., Phillips, G., Pulk, R., Rawson, T.E., Ultsch, M., Walker, L., Wiesmann, C., Zhang, B., Zhu, B.Y., Cochran, A.G.(2009) J Med Chem 52: 3300-3307
- PubMed: 19402633
- DOI: https://doi.org/10.1021/jm9000314
- Primary Citation of Related Structures:
3H0Y, 3H0Z, 3H10 - PubMed Abstract:
The two major Aurora kinases carry out critical functions at distinct mitotic stages. Selective inhibitors of these kinases, as well as pan-Aurora inhibitors, show antitumor efficacy and are now under clinical investigation. However, the ATP-binding sites of Aurora A and Aurora B are virtually identical, and the structural basis for selective inhibition has therefore not been clear. We report here a class of bisanilinopyrimidine Aurora A inhibitors with excellent selectivity for Aurora A over Aurora B, both in enzymatic assays and in cellular phenotypic assays. Crystal structures of two of the inhibitors in complex with Aurora A implicate a single amino acid difference in Aurora B as responsible for poor inhibitory activity against this enzyme. Mutation of this residue in Aurora B (E161T) or Aurora A (T217E) is sufficient to swap the inhibition profile, suggesting that this difference might be exploited more generally to achieve high selectivity for Aurora A.
Organizational Affiliation:
Department of Small Molecule Drug Discovery, Genentech, Inc., 1 DNA Way, South San Francisco, California 94080, USA.