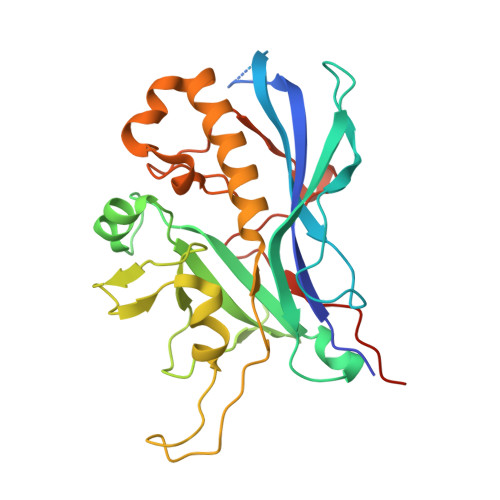
Structural conservation of the myoviridae phage tail sheath protein fold.
Aksyuk, A.A., Kurochkina, L.P., Fokine, A., Forouhar, F., Mesyanzhinov, V.V., Tong, L., Rossmann, M.G.(2011) Structure 19: 1885-1894
- PubMed: 22153511
- DOI: https://doi.org/10.1016/j.str.2011.09.012
- Primary Citation of Related Structures:
3J0H, 3J0I, 3SPE - PubMed Abstract:
Bacteriophage phiKZ is a giant phage that infects Pseudomonas aeruginosa, a human pathogen. The phiKZ virion consists of a 1450 Å diameter icosahedral head and a 2000 Å-long contractile tail. The structure of the whole virus was previously reported, showing that its tail organization in the extended state is similar to the well-studied Myovirus bacteriophage T4 tail. The crystal structure of a tail sheath protein fragment of phiKZ was determined to 2.4 Å resolution. Furthermore, crystal structures of two prophage tail sheath proteins were determined to 1.9 and 3.3 Å resolution. Despite low sequence identity between these proteins, all of these structures have a similar fold. The crystal structure of the phiKZ tail sheath protein has been fitted into cryo-electron-microscopy reconstructions of the extended tail sheath and of a polysheath. The structural rearrangement of the phiKZ tail sheath contraction was found to be similar to that of phage T4.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, West Lafayette, IN 47907-2032, USA.