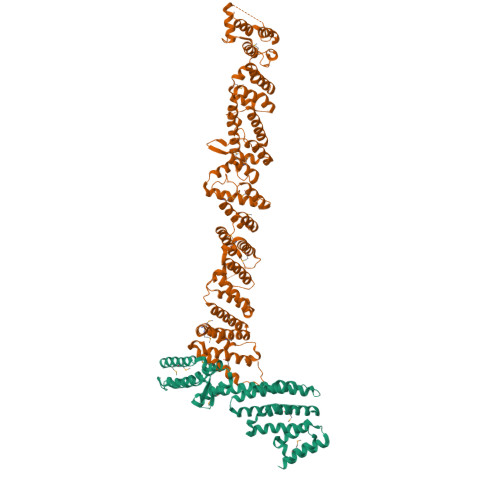
A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1.
Ren, Y., Yip, C.K., Tripathi, A., Huie, D., Jeffrey, P.D., Walz, T., Hughson, F.M.(2009) Cell 139: 1119-1129
- PubMed: 20005805
- DOI: https://doi.org/10.1016/j.cell.2009.11.002
- Primary Citation of Related Structures:
3K8P - PubMed Abstract:
Vesicle trafficking requires membrane fusion, mediated by SNARE proteins, and upstream events that probably include "tethering," an initial long-range attachment between a vesicle and its target organelle. Among the factors proposed to mediate tethering are a set of multisubunit tethering complexes (MTCs). The Dsl1 complex, with only three subunits, is the simplest known MTC and is essential for the retrograde traffic of COPI-coated vesicles from the Golgi to the ER. To elucidate structural principles underlying MTC function, we have determined the structure of the Dsl1 complex, revealing a tower containing at its base the binding sites for two ER SNAREs and at its tip a flexible lasso for capturing vesicles. The Dsl1 complex binds to individual SNAREs via their N-terminal regulatory domains and also to assembled SNARE complexes; moreover, it is capable of accelerating SNARE complex assembly. Our results suggest that even the simplest MTC may be capable of orchestrating vesicle capture, uncoating, and fusion.
Organizational Affiliation:
Department of Molecular Biology, Princeton University, Princeton, NJ 08544, USA.