Structural basis of the substrate specificity of Bacillus cereus adenosine phosphorylase.
Dessanti, P., Zhang, Y., Allegrini, S., Tozzi, M.G., Sgarrella, F., Ealick, S.E.(2012) Acta Crystallogr D Biol Crystallogr 68: 239-248
- PubMed: 22349225
- DOI: https://doi.org/10.1107/S090744491200073X
- Primary Citation of Related Structures:
3UAV, 3UAW, 3UAX, 3UAY, 3UAZ - PubMed Abstract:
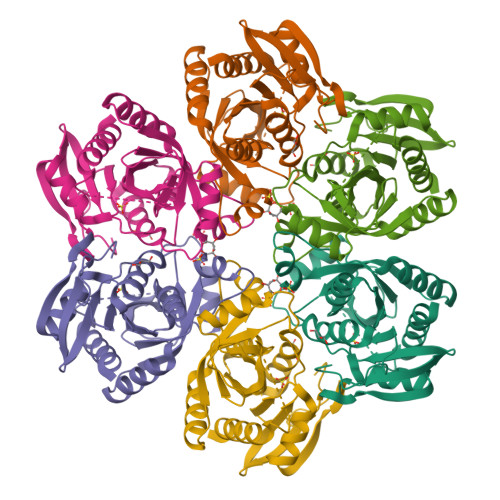
Purine nucleoside phosphorylases catalyze the phosphorolytic cleavage of the glycosidic bond of purine (2'-deoxy)nucleosides, generating the corresponding free base and (2'-deoxy)-ribose 1-phosphate. Two classes of PNPs have been identified: homotrimers specific for 6-oxopurines and homohexamers that accept both 6-oxopurines and 6-aminopurines. Bacillus cereus adenosine phosphorylase (AdoP) is a hexameric PNP; however, it is highly specific for 6-aminopurines. To investigate the structural basis for the unique substrate specificity of AdoP, the active-site mutant D204N was prepared and kinetically characterized and the structures of the wild-type protein and the D204N mutant complexed with adenosine and sulfate or with inosine and sulfate were determined at high resolution (1.2-1.4 Å). AdoP interacts directly with the preferred substrate through a hydrogen-bond donation from the catalytically important residue Asp204 to N7 of the purine base. Comparison with Escherichia coli PNP revealed a more optimal orientation of Asp204 towards N7 of adenosine and a more closed active site. When inosine is bound, two water molecules are interposed between Asp204 and the N7 and O6 atoms of the nucleoside, thus allowing the enzyme to find alternative but less efficient ways to stabilize the transition state. The mutation of Asp204 to asparagine led to a significant decrease in catalytic efficiency for adenosine without affecting the efficiency of inosine cleavage.
Organizational Affiliation:
Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853-1301, USA.