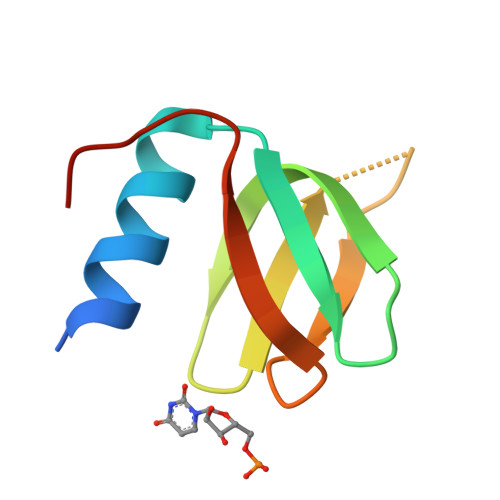
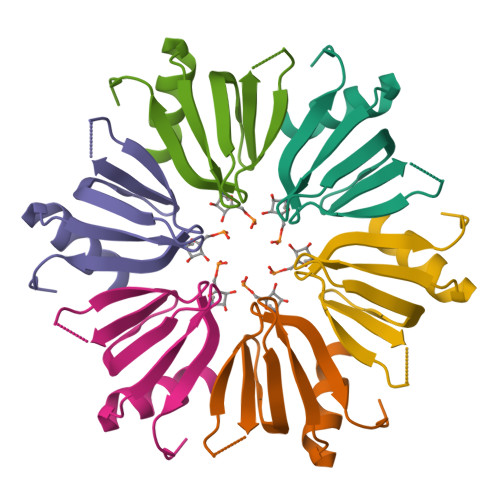
Structure of an Escherichia coli Hfq:RNA complex at 0.97 angstrom resolution.
Schulz, E.C., Barabas, O.(2014) Acta Crystallogr F Struct Biol Commun 70: 1492-1497
- PubMed: 25372815
- DOI: https://doi.org/10.1107/S2053230X14020044
- Primary Citation of Related Structures:
4PNO - PubMed Abstract:
In bacteria, small RNAs (sRNAs) silence or activate target genes through base pairing with the mRNA, thereby modulating its translation. A central player in this process is the RNA chaperone Hfq, which facilitates the annealing of sRNAs with their target mRNAs. Hfq has two RNA-binding surfaces that recognize A-rich and U-rich sequences, and is believed to bind an sRNA-mRNA pair simultaneously. However, how Hfq promotes annealing remains unclear. Here, the crystal structure of Escherichia coli Hfq is presented in complex with U6-RNA bound to its proximal binding site at 0.97 Å resolution, revealing the Hfq-RNA interaction in exceptional detail.
Organizational Affiliation:
Structural and Computational Biology Unit, European Molecular Biology Laboratory, Meyerhofstrasse 1, 69117 Heidelberg, Germany.