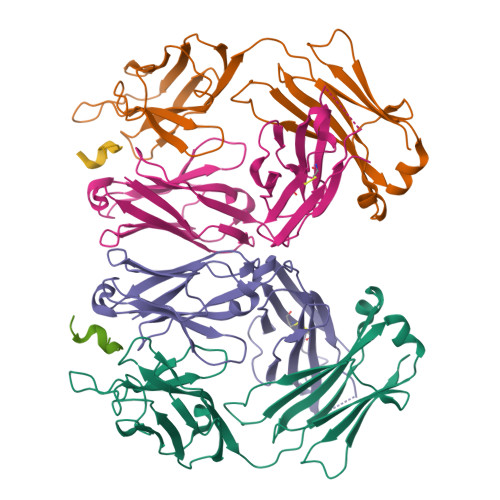
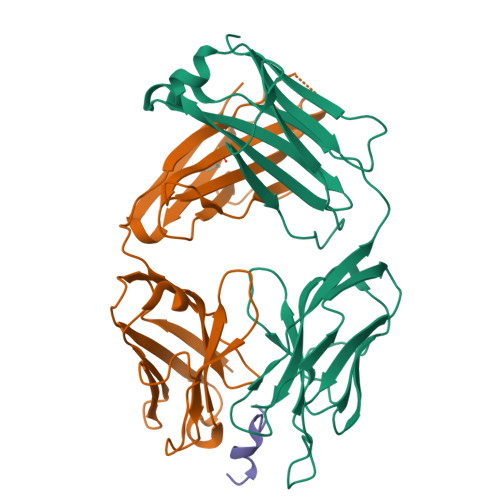
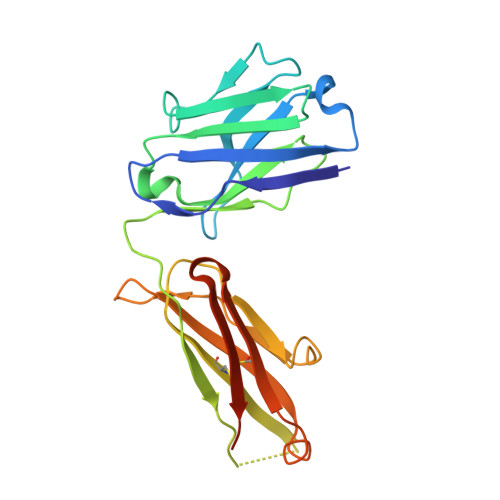
Molecular basis for mid-region amyloid-beta capture by leading Alzheimer's disease immunotherapies.
Crespi, G.A., Hermans, S.J., Parker, M.W., Miles, L.A.(2015) Sci Rep 5: 9649-9649
- PubMed: 25880481
- DOI: https://doi.org/10.1038/srep09649
- Primary Citation of Related Structures:
4XXD - PubMed Abstract:
Solanezumab (Eli Lilly) and crenezumab (Genentech) are the leading clinical antibodies targeting Amyloid-β (Aβ) to be tested in multiple Phase III clinical trials for the prevention of Alzheimer's disease in at-risk individuals. Aβ capture by these clinical antibodies is explained here with the first reported mid-region Aβ-anti-Aβ complex crystal structure. Solanezumab accommodates a large Aβ epitope (960 Å(2) buried interface over residues 16 to 26) that forms extensive contacts and hydrogen bonds to the antibody, largely via main-chain Aβ atoms and a deeply buried Phe19-Phe20 dipeptide core. The conformation of Aβ captured is an intermediate between observed sheet and helical forms with intramolecular hydrogen bonds stabilising residues 20-26 in a helical conformation. Remarkably, Aβ-binding residues are almost perfectly conserved in crenezumab. The structure explains the observed shared cross reactivity of solanezumab and crenezumab with proteins abundant in plasma that exhibit this Phe-Phe dipeptide.
Organizational Affiliation:
ACRF Rational Drug Discovery Centre, St. Vincent's Institute of Medical Research, Fitzroy, Victoria 3065, Australia.