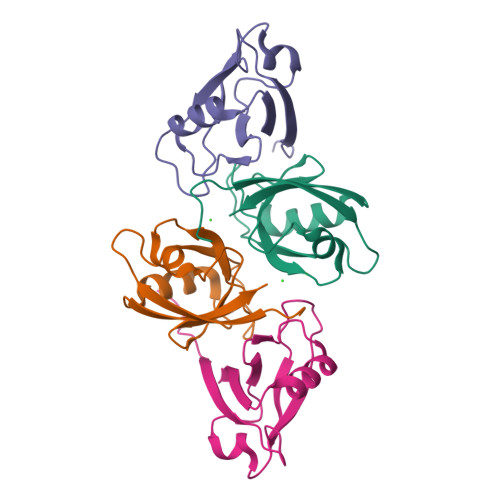
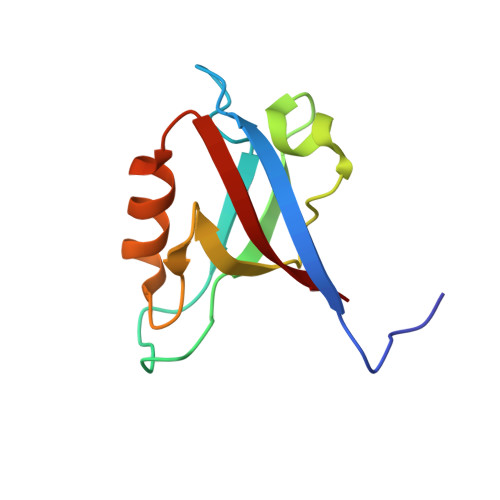
High-resolution crystal structure of the PDZ1 domain of human protein tyrosine phosphatase PTP-Bas.
Lee, S.O., Lee, M.K., Ku, B., Bae, K.H., Lee, S.C., Lim, H.M., Kim, S.J., Chi, S.W.(2016) Biochem Biophys Res Commun 478: 1205-1210
- PubMed: 27544031
- DOI: https://doi.org/10.1016/j.bbrc.2016.08.095
- Primary Citation of Related Structures:
5GLJ - PubMed Abstract:
Protein tyrosine phosphatase-Basophil (PTP-Bas) is a membrane-associated protein tyrosine phosphatase with five PDZ domains and is involved in apoptosis, tumorigenesis, and insulin signaling. The interaction between PTP-Bas and tandem-PH-domain-containing protein 1/2 (TAPP1/2) plays an essential role in the regulation of insulin signaling. Despite its high sequence homology with the other PDZ domains, only the PDZ1 domain of PTP-Bas showed distinct binding specificity for TAPP1/2. Although the interaction between PTP-Bas PDZ1 and TAPP1/2 is a therapeutic target for diabetes, the structural basis for the interaction has not been elucidated. In the present study, we determined the crystal structure of the PTP-Bas PDZ1 domain at 1.6 Å resolution. In addition, we calculated the structural models of complexes of PTP-Bas PDZ1 and the C-terminal peptides of TAPP1/2 (referred to as TAPP1p/2p). Structural comparison with the PTP-Bas PDZ2/RA-GEF2 peptide complex revealed a structural basis for distinct binding specificity of PTP-Bas PDZ1 for TAPP1p/2p peptides. Our high-resolution crystal structure of PTP-Bas PDZ1 will serve as a useful template for rational structure-based design of novel anti-diabetes therapeutics.
Organizational Affiliation:
Disease Target Structure Research Center, KRIBB, Daejeon 34141, Republic of Korea; Department of Biological Sciences, College of Biological Sciences and Biotechnology, Chungnam National University, Daejeon 34134, Republic of Korea.