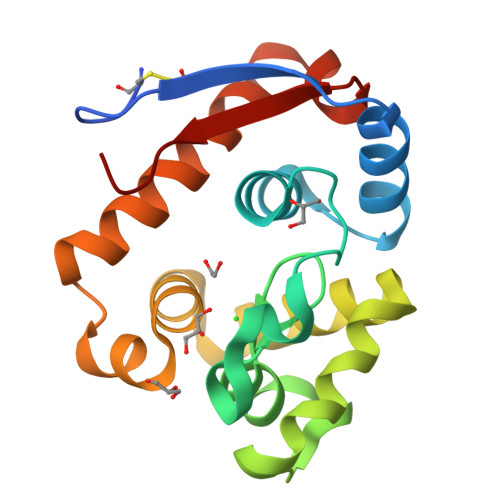
A beta-Mannanase with a Lysozyme-like Fold and a Novel Molecular Catalytic Mechanism.
Jin, Y., Petricevic, M., John, A., Raich, L., Jenkins, H., Portela De Souza, L., Cuskin, F., Gilbert, H.J., Rovira, C., Goddard-Borger, E.D., Williams, S.J., Davies, G.J.(2016) ACS Cent Sci 2: 896-903
- PubMed: 28058278
- DOI: https://doi.org/10.1021/acscentsci.6b00232
- Primary Citation of Related Structures:
5JTS, 5JU9, 5JUG - PubMed Abstract:
The enzymatic cleavage of β-1,4-mannans is achieved by endo -β-1,4-mannanases, enzymes involved in germination of seeds and microbial hemicellulose degradation, and which have increasing industrial and consumer product applications. β-Mannanases occur in a range of families of the CAZy sequence-based glycoside hydrolase (GH) classification scheme including families 5, 26, and 113. In this work we reveal that β-mannanases of the newly described GH family 134 differ from other mannanase families in both their mechanism and tertiary structure. A representative GH family 134 endo -β-1,4-mannanase from a Streptomyces sp. displays a fold closely related to that of hen egg white lysozyme but acts with inversion of stereochemistry. A Michaelis complex with mannopentaose, and a product complex with mannotriose, reveal ligands with pyranose rings distorted in an unusual inverted chair conformation. Ab initio quantum mechanics/molecular mechanics metadynamics quantified the energetically accessible ring conformations and provided evidence in support of a 1 C 4 → 3 H 4 ‡ → 3 S 1 conformational itinerary along the reaction coordinate. This work, in concert with that on GH family 124 cellulases, reveals how the lysozyme fold can be co-opted to catalyze the hydrolysis of different polysaccharides in a mechanistically distinct manner.
Organizational Affiliation:
York Structural Biology Laboratory, Department of Chemistry, University of York , Heslington, YO10 5DD, U.K.