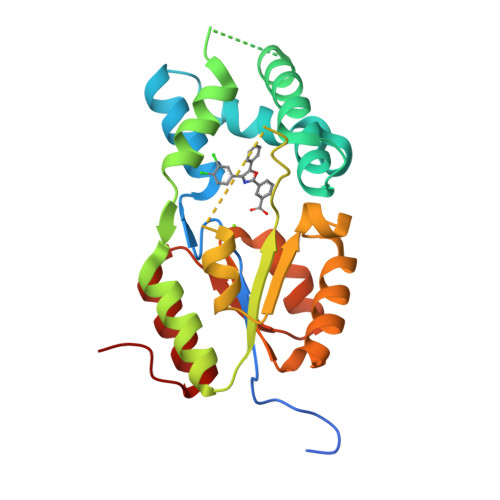
Discovery of first in vivo active inhibitors of soluble epoxide hydrolase (sEH) phosphatase domain.
Kramer, J.S., Woltersdorf, S., Duflot, T., Hiesinger, K., Lillich, F.F., Knoll, F., Wittmann, S.K., Klingler, F.M., Brunst, S., Chaikuad, A., Morisseau, C., Hammock, B.D., Buccellati, C., Sala, A., Rovati, G.E., Leuillier, M., Fraineau, S., Rondeaux, J., Hernandez Olmos, V., Heering, J., Merk, D., Pogoryelov, D., Steinhilber, D., Knapp, S., Bellien, J., Proschak, E.(2019) J Med Chem
- PubMed: 31436984
- DOI: https://doi.org/10.1021/acs.jmedchem.9b00445
- Primary Citation of Related Structures:
5MWA - PubMed Abstract:
The emerging pharmacological target soluble epoxide hydrolase (sEH) is a bifunctional enzyme exhibiting two different catalytic activities that are located in two distinct domains. Although the physiological role of the C-terminal hydrolase domain is well-investigated, little is known about its phosphatase activity, located in the N-terminal phosphatase domain of sEH (sEH-P). Herein we report the discovery and optimization of the first inhibitor of human and rat sEH-P that is applicable in vivo. X-ray structure analysis of the sEH phosphatase domain complexed with an inhibitor provides insights in the molecular basis of small-molecule sEH-P inhibition and helps to rationalize the structure-activity relationships. 4-(4-(3,4-Dichlorophenyl)-5-phenyloxazol-2-yl)butanoic acid ( 22b , SWE101) has an excellent pharmacokinetic and pharmacodynamic profile in rats and enables the investigation of the physiological and pathophysiological role of sEH-P in vivo.
Organizational Affiliation:
Institute of Pharmaceutical Chemistry , Goethe-University Frankfurt , Max-von-Laue-Strasse 9 , 60438 Frankfurt am Main , Germany.