Biochemical and Structural Analysis of FomD That Catalyzes the Hydrolysis of Cytidylyl ( S)-2-Hydroxypropylphosphonate in Fosfomycin Biosynthesis.
Sato, S., Miyanaga, A., Kim, S.Y., Kuzuyama, T., Kudo, F., Eguchi, T.(2018) Biochemistry 57: 4858-4866
- PubMed: 30010320
- DOI: https://doi.org/10.1021/acs.biochem.8b00690
- Primary Citation of Related Structures:
5ZDM, 5ZDN - PubMed Abstract:
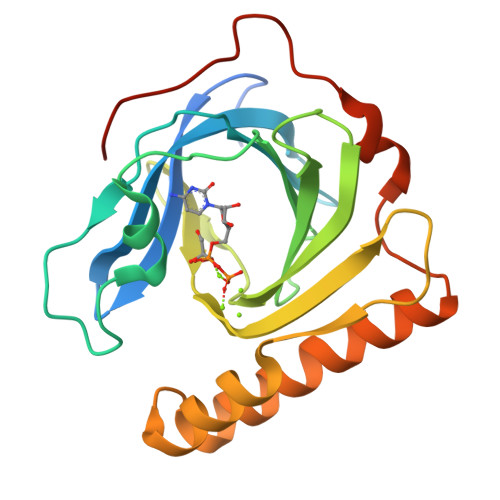
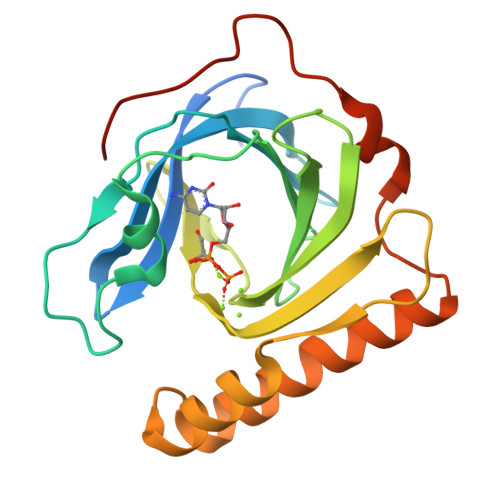
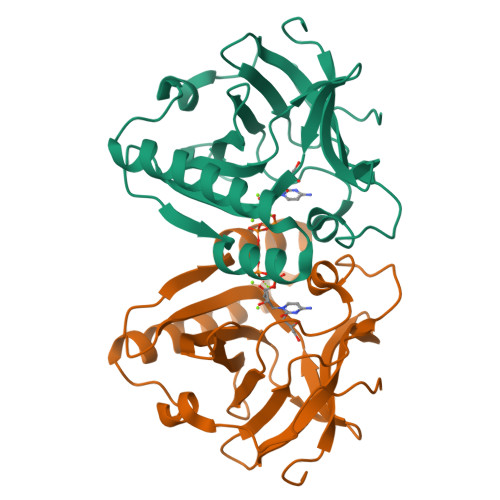
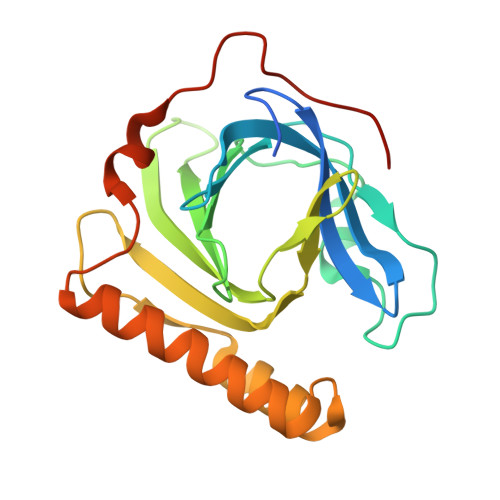
In fosfomycin biosynthesis, the hydrolysis of cytidylyl ( S)-2-hydroxypropylphosphonate [( S)-HPP-CMP] to afford ( S)-HPP is the only uncharacterized step. Because FomD is an uncharacterized protein with a DUF402 domain that is encoded in the fosfomycin biosynthetic gene cluster, FomD was hypothesized to be responsible for this reaction. In this study, FomD was found to hydrolyze ( S)-HPP-CMP to give ( S)-HPP and CMP efficiently in the presence of Mn 2+ or Co 2+ . FomD also hydrolyzed cytidylyl 2-hydroxyethylphosphonate (HEP-CMP), which is a biosynthetic intermediate before C-methylation. The k cat / K M value of FomD with ( S)-HPP-CMP was 10-fold greater than that with HEP-CMP, suggesting that FomD hydrolyzes ( S)-HPP-CMP rather than HEP-CMP in bacteria. The crystal structure of FomD showed that this protein adopts a barrel-like fold, which consists of a large twisted antiparallel β-sheet. This is a key structural feature of the DUF402 domain-containing proteins. Two metal cations are located between the FomD barrel and the two α-helices at the C-terminus and serve to presumably activate the phosphonate group of substrates for hydrolysis. Docking simulations with ( S)-HPP-CMP suggested that the methyl group at the C2 position of the HPP moiety is recognized by a hydrophobic interaction with Trp68. Further mutational analysis suggested that a conserved Tyr107 among the DUF402 domain family of proteins activates a water molecule to promote nucleophilic attack on the phosphorus atom of the phosphonate moiety. These findings provide mechanistic insights into the FomD reaction and lead to a complete understanding of the fosfomycin biosynthetic pathway in Streptomyces.
Organizational Affiliation:
Department of Chemistry , Tokyo Institute of Technology , 2-12-1 O-okayama , Meguro-ku, Tokyo 152-8551 , Japan.