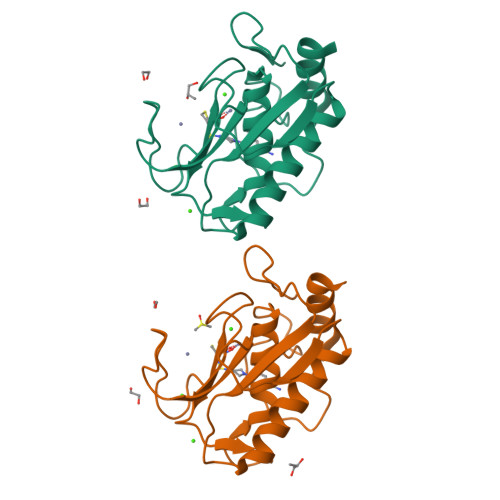
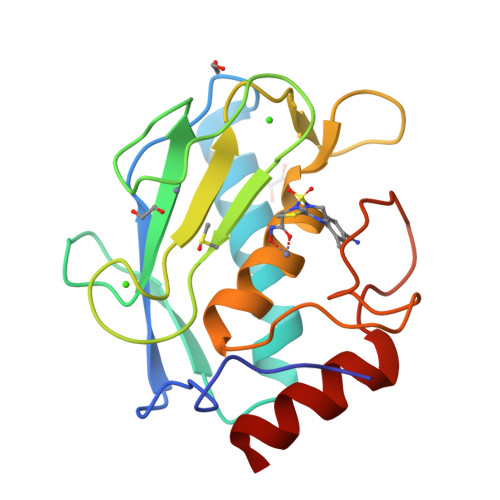
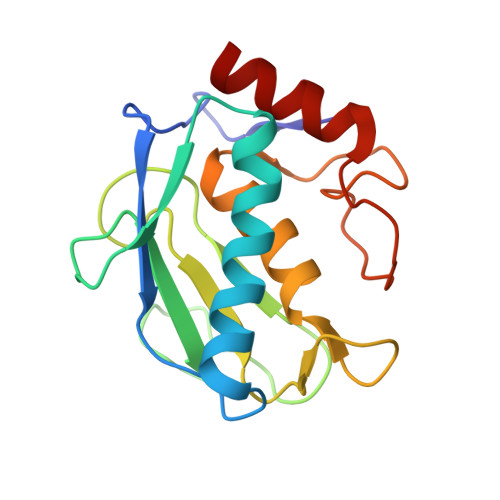
Discovery of a new selective inhibitor of A Disintegrin And Metalloprotease 10 (ADAM-10) able to reduce the shedding of NKG2D ligands in Hodgkin's lymphoma cell models.
Camodeca, C., Nuti, E., Tepshi, L., Boero, S., Tuccinardi, T., Stura, E.A., Poggi, A., Zocchi, M.R., Rossello, A.(2016) Eur J Med Chem 111: 193-201
- PubMed: 26871660
- DOI: https://doi.org/10.1016/j.ejmech.2016.01.053
- Primary Citation of Related Structures:
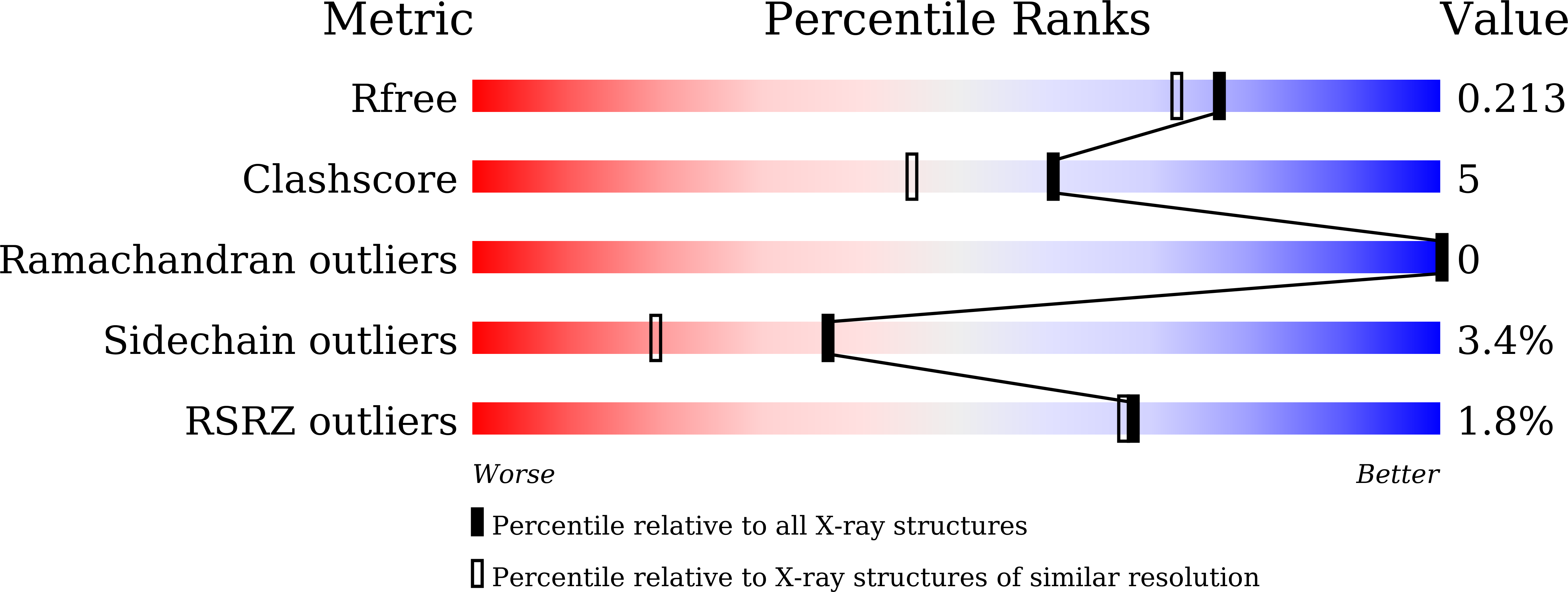
5CUH - PubMed Abstract:
Hodgkin's lymphoma (HL) is the most common malignant lymphoma in young adults in the western world. This disease is characterized by an overexpression of ADAM-10 with increased release of NKG2D ligands, involved in an impaired immune response against tumor cells. We designed and synthesized two new ADAM-10 selective inhibitors, 2 and 3 based on previously published ADAM-17 selective inhibitor 1. The most promising compound was the thiazolidine derivative 3, with nanomolar activity for ADAM-10, high selectivity over ADAM-17 and MMPs and good efficacy in reducing the shedding of NKG2D ligands (MIC-B and ULBP3) in three different HL cell lines at non-toxic doses. Molecular modeling studies were used to drive the design and X-ray crystallography studies were carried out to explain the selectivity of 3 for ADAM-10 over MMPs.
Organizational Affiliation:
Division of Immunology, Transplants and Infectious Diseases, San Raffaele Scientific Institute, 20132 Milan, Italy.