Small-Molecule Covalent Modification of Conserved Cysteine Leads to Allosteric Inhibition of the TEAD⋅Yap Protein-Protein Interaction.
Bum-Erdene, K., Zhou, D., Gonzalez-Gutierrez, G., Ghozayel, M.K., Si, Y., Xu, D., Shannon, H.E., Bailey, B.J., Corson, T.W., Pollok, K.E., Wells, C.D., Meroueh, S.O.(2019) Cell Chem Biol 26: 378
- PubMed: 30581134
- DOI: https://doi.org/10.1016/j.chembiol.2018.11.010
- Primary Citation of Related Structures:
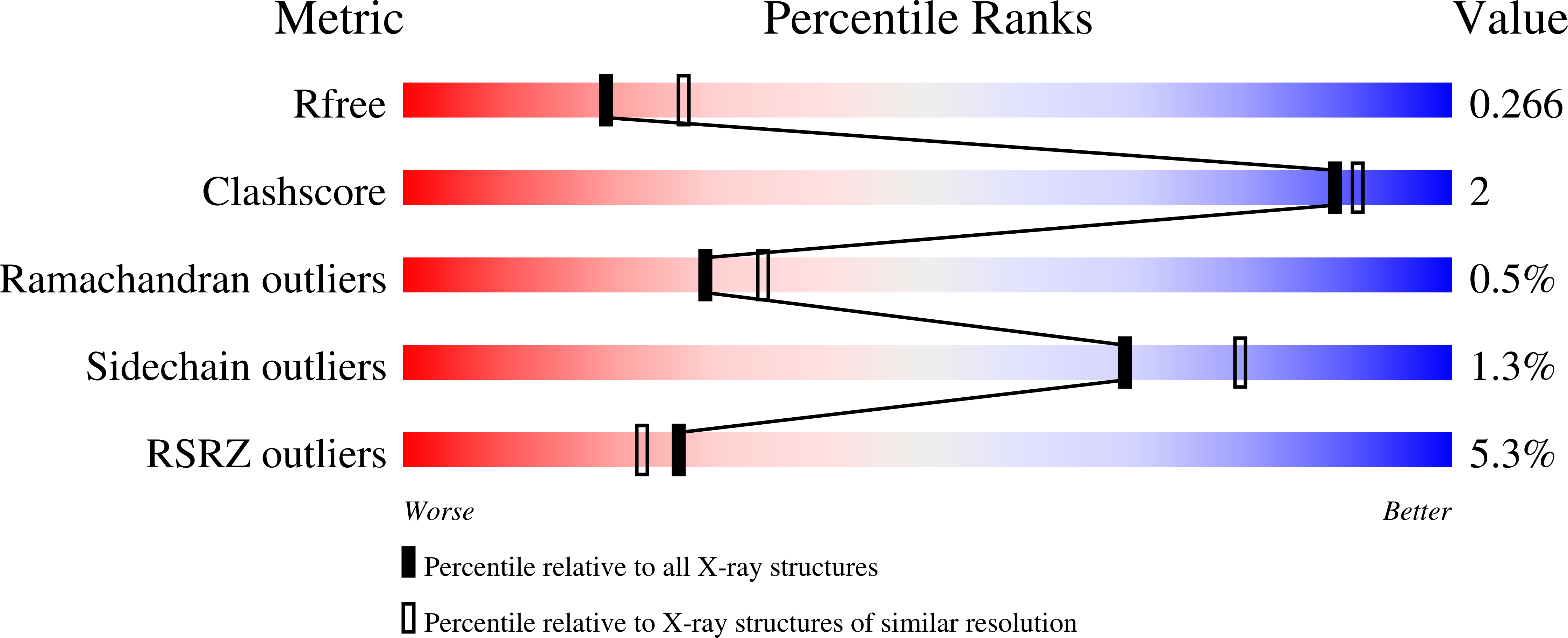
6E5G - PubMed Abstract:
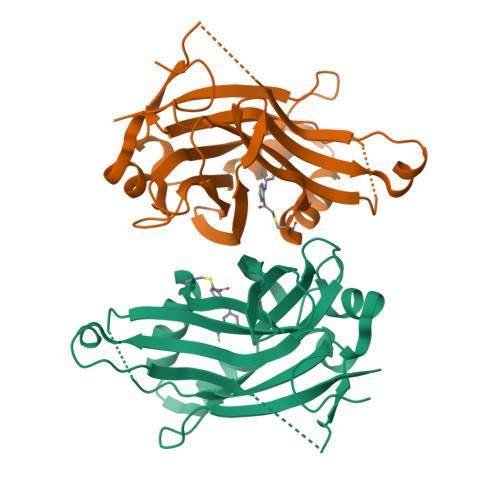
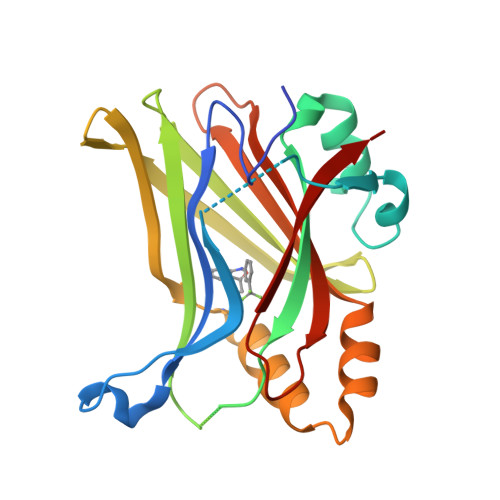
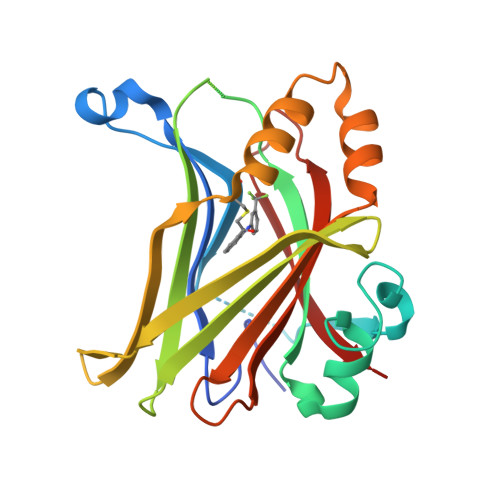
The Hippo pathway coordinates extracellular signals onto the control of tissue homeostasis and organ size. Hippo signaling primarily regulates the ability of Yap1 to bind and co-activate TEA domain (TEAD) transcription factors. Yap1 tightly binds to TEAD4 via a large flat interface, making the development of small-molecule orthosteric inhibitors highly challenging. Here, we report small-molecule TEAD⋅Yap inhibitors that rapidly and selectively form a covalent bond with a conserved cysteine located within the unique deep hydrophobic palmitate-binding pocket of TEADs. Inhibition of TEAD4 binding to Yap1 by these compounds was irreversible and occurred on a longer time scale. In mammalian cells, the compounds formed a covalent complex with TEAD4, inhibited its binding to Yap1, blocked its transcriptional activity, and suppressed expression of connective tissue growth factor. The compounds inhibited cell viability of patient-derived glioblastoma spheroids, making them suitable as chemical probes to explore Hippo signaling in cancer.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN 46202, USA.