Genetic Engineering of an Artificial Metalloenzyme for Transfer Hydrogenation of a Self-Immolative Substrate in Escherichia coli's Periplasm.
Zhao, J., Rebelein, J.G., Mallin, H., Trindler, C., Pellizzoni, M.M., Ward, T.R.(2018) J Am Chem Soc 140: 13171-13175
- PubMed: 30272972
- DOI: https://doi.org/10.1021/jacs.8b07189
- Primary Citation of Related Structures:
6GMI - PubMed Abstract:
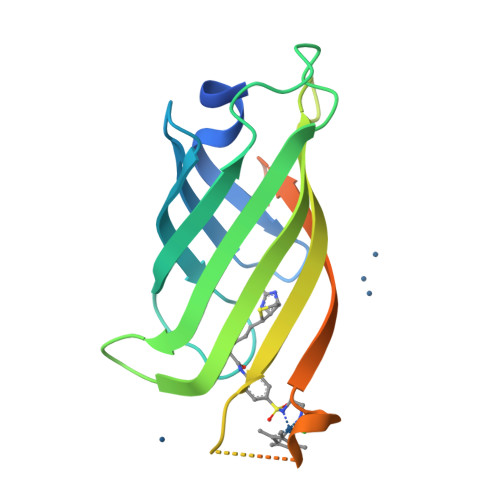
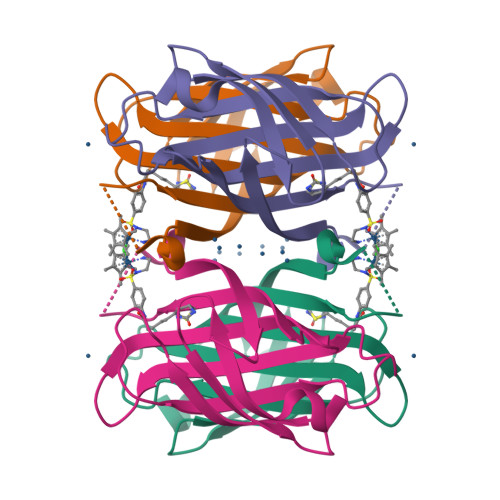
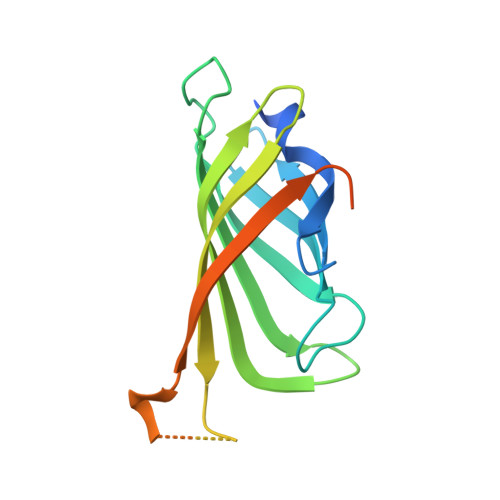
Artificial metalloenzymes (ArMs), which combine an abiotic metal cofactor with a protein scaffold, catalyze various synthetically useful transformations. To complement the natural enzymes' repertoire, effective optimization protocols to improve ArM's performance are required. Here we report on our efforts to optimize the activity of an artificial transfer hydrogenase (ATHase) using Escherichia coli whole cells. For this purpose, we rely on a self-immolative quinolinium substrate which, upon reduction, releases fluorescent umbelliferone, thus allowing efficient screening. Introduction of a loop in the immediate proximity of the Ir-cofactor afforded an ArM with up to 5-fold increase in transfer hydrogenation activity compared to the wild-type ATHase using purified mutants.
Organizational Affiliation:
Department of Chemistry , University of Basel , Mattenstrasse 24a, BPR 1096 , CH-4058 Basel , Switzerland.