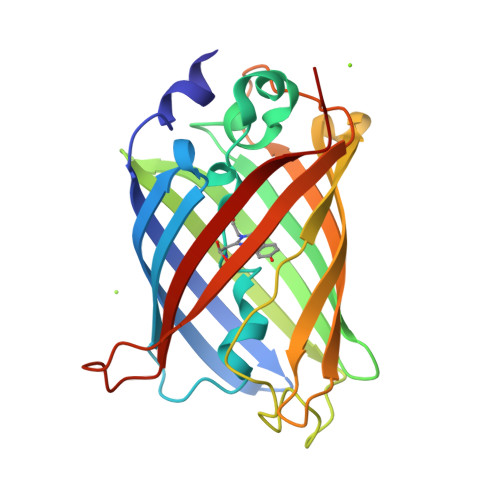
Subatomic resolution X-ray structures of green fluorescent protein.
Takaba, K., Tai, Y., Eki, H., Dao, H.A., Hanazono, Y., Hasegawa, K., Miki, K., Takeda, K.(2019) IUCrJ 6: 387-400
- PubMed: 31098020
- DOI: https://doi.org/10.1107/S205225251900246X
- Primary Citation of Related Structures:
6JGH, 6JGI, 6JGJ - PubMed Abstract:
Green fluorescent protein (GFP) is a light-emitting protein that does not require a prosthetic group for its fluorescent activity. As such, GFP has become indispensable as a molecular tool in molecular biology. Nonetheless, there has been no subatomic elucidation of the GFP structure owing to the structural polymorphism around the chromophore. Here, subatomic resolution X-ray structures of GFP without the structural polymorphism are reported. The positions of H atoms, hydrogen-bonding network patterns and accurate geometric parameters were determined for the two protonated forms. Compared with previously determined crystal structures and theoretically optimized structures, the anionic chromophores of the structures represent the authentic resonance state of GFP. In addition, charge-density analysis based on atoms-in-molecules theory and noncovalent interaction analysis highlight weak but substantial interactions between the chromophore and the protein environment. Considered with the derived chemical indicators, the lone pair-π interactions between the chromophore and Thr62 should play a sufficient role in maintaining the electronic state of the chromophore. These results not only reveal the fine structural features that are critical to understanding the properties of GFP, but also highlight the limitations of current quantum-chemical calculations.
Organizational Affiliation:
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan.