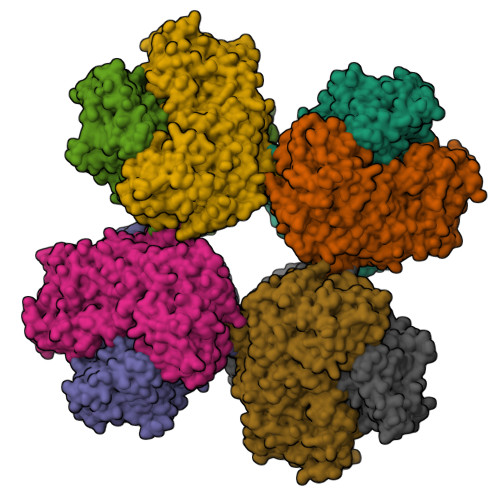
Ambient temperature structure of phosphoketolase from Bifidobacterium longum determined by serial femtosecond X-ray crystallography.
Nakata, K., Kashiwagi, T., Kunishima, N., Naitow, H., Matsuura, Y., Miyano, H., Mizukoshi, T., Tono, K., Yabashi, M., Nango, E., Iwata, S.(2023) Acta Crystallogr D Struct Biol 79: 290-303
- PubMed: 36974963
- DOI: https://doi.org/10.1107/S2059798323001638
- Primary Citation of Related Structures:
7C8H, 7C8I - PubMed Abstract:
Phosphoketolase and transketolase are thiamine diphosphate-dependent enzymes and play a central role in the primary metabolism of bifidobacteria: the bifid shunt. The enzymes both catalyze phosphorolytic cleavage of xylulose 5-phosphate or fructose 6-phosphate in the first reaction step, but possess different substrate specificity in the second reaction step, where phosphoketolase and transketolase utilize inorganic phosphate (P i ) and D-ribose 5-phosphate, respectively, as the acceptor substrate. Structures of Bifidobacterium longum phosphoketolase holoenzyme and its complex with a putative inhibitor, phosphoenolpyruvate, were determined at 2.5 Å resolution by serial femtosecond crystallography using an X-ray free-electron laser. In the complex structure, phosphoenolpyruvate was present at the entrance to the active-site pocket and plugged the channel to thiamine diphosphate. The phosphate-group position of phosphoenolpyruvate coincided well with those of xylulose 5-phosphate and fructose 6-phosphate in the structures of their complexes with transketolase. The most striking structural change was observed in a loop consisting of Gln546-Asp547-His548-Asn549 (the QN-loop) at the entrance to the active-site pocket. Contrary to the conformation of the QN-loop that partially covers the entrance to the active-site pocket (`closed form') in the known crystal structures, including the phosphoketolase holoenzyme and its complexes with reaction intermediates, the QN-loop in the current ambient structures showed a more compact conformation with a widened entrance to the active-site pocket (`open form'). In the phosphoketolase reaction, the `open form' QN-loop may play a role in providing the binding site for xylulose 5-phosphate or fructose 6-phosphate in the first step, and the `closed form' QN-loop may help confer specificity for P i in the second step.
Organizational Affiliation:
Research Institute for Bioscience Products and Fine Chemicals, Ajinomoto Co. Inc., 1-1 Suzuki-cho, Kawasaki-ku, Kawasaki 210-8681, Japan.