MANORAA: A machine learning platform to guide protein-ligand design by anchors and influential distances.
Tanramluk, D., Pakotiprapha, D., Phoochaijaroen, S., Chantravisut, P., Thampradid, S., Vanichtanankul, J., Narupiyakul, L., Akavipat, R., Yuvaniyama, J.(2022) Structure 30: 181-189.e5
- PubMed: 34614393
- DOI: https://doi.org/10.1016/j.str.2021.09.004
- Primary Citation of Related Structures:
7F3Y, 7F3Z - PubMed Abstract:
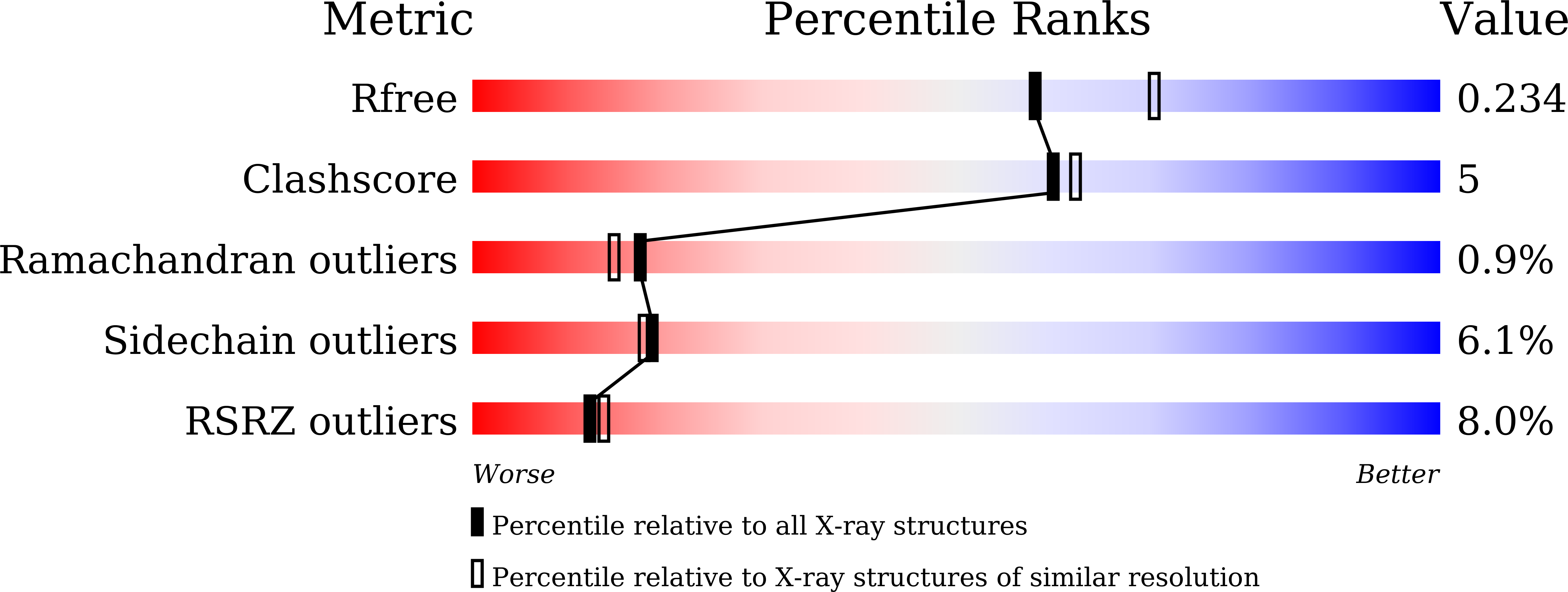
The MANORAA platform uses structure-based approaches to provide information on drug design originally derived from mapping tens of thousands of amino acids on a grid. In-depth analyses of the pockets, frequently occurring atoms, influential distances, and active-site boundaries are used for the analysis of active sites. The algorithms derived provide model equations that can predict whether changes in distances, such as contraction or expansion, will result in improved binding affinity. The algorithm is confirmed using kinetic studies of dihydrofolate reductase (DHFR), together with two DHFR-TS crystal structures. Empirical analyses of 881 crystal structures involving 180 ligands are used to interpret protein-ligand binding affinities. MANORAA links to major biological databases for web-based analysis of drug design. The frequency of atoms inside the main protease structures, including those from SARS-CoV-2, shows how the rigid part of the ligand can be used as a probe for molecular design (http://manoraa.org).
Organizational Affiliation:
Institute of Molecular Biosciences, Mahidol University, Salaya, Nakhon Pathom 73170, Thailand; Integrative Computational BioScience (ICBS) Center, Mahidol University, Salaya, Nakhon Pathom 73170, Thailand. Electronic address: [email protected].