Macrocyclic Retinoic Acid Receptor-Related Orphan Receptor C2 Inverse Agonists.
Schnute, M.E., Trujillo, J.I., Lee, K.L., Unwalla, R., Vajdos, F.F., Kauppi, B., Nuhant, P., Flick, A.C., Crouse, K.K., Zhao, Y., Samuel, A., Lombardo, V., Taylor, A.P., Brault, A.L., Knafels, J.D., Vazquez, M.L., Berstein, G.(2023) ACS Med Chem Lett 14: 191-198
- PubMed: 36793423
- DOI: https://doi.org/10.1021/acsmedchemlett.2c00500
- Primary Citation of Related Structures:
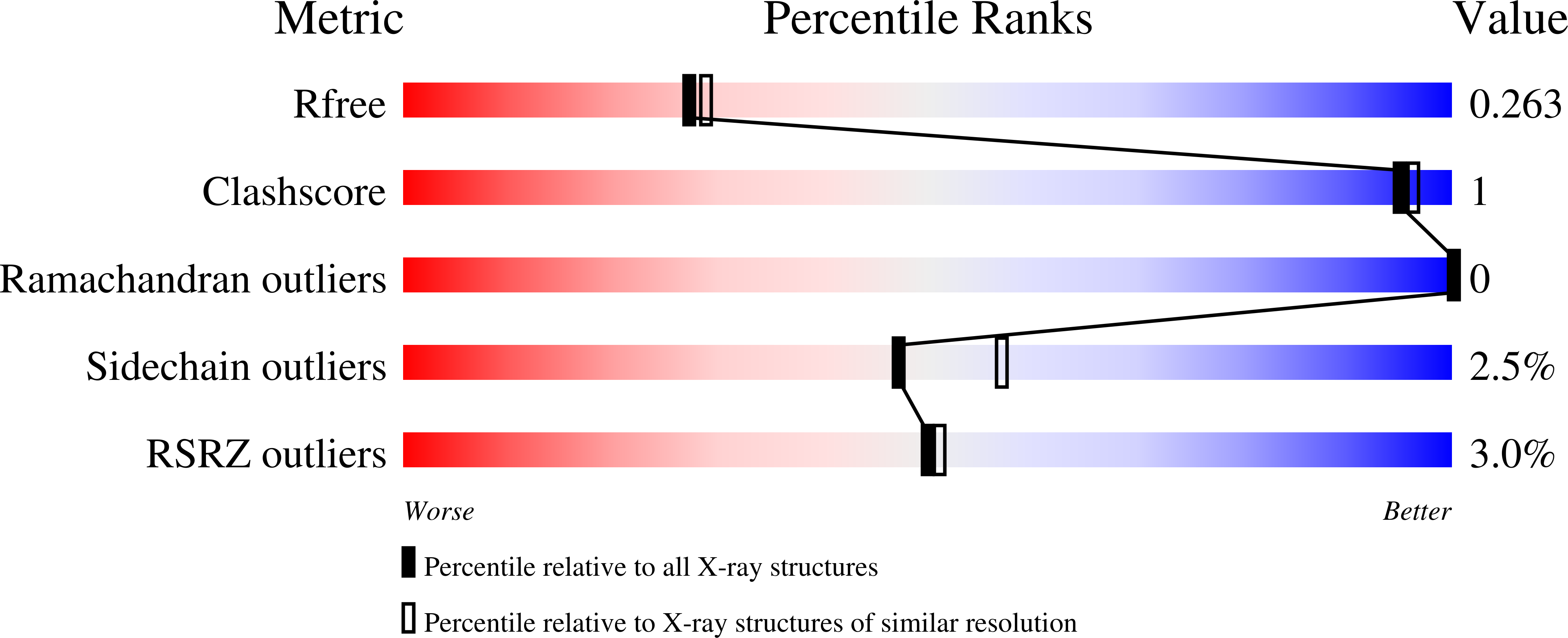
8FAV, 8FB1, 8FB2 - PubMed Abstract:
Macrocyclic retinoic acid receptor-related orphan receptor C2 (RORC2) inverse agonists have been designed with favorable properties for topical administration. Inspired by the unanticipated bound conformation of an acyclic sulfonamide-based RORC2 ligand from cocrystal structure analysis, macrocyclic linker connections between the halves of the molecule were explored. Further optimization of analogues was accomplished to maximize potency and refine physiochemical properties (MW, lipophilicity) best suited for topical application. Compound 14 demonstrated potent inhibition of interleukin-17A (IL-17A) production by human Th17 cells and in vitro permeation through healthy human skin achieving high total compound concentration in both skin epidermis and dermis layers.
Organizational Affiliation:
Medicine Design, Pfizer Inc., Cambridge, Massachusetts 02139, United States.