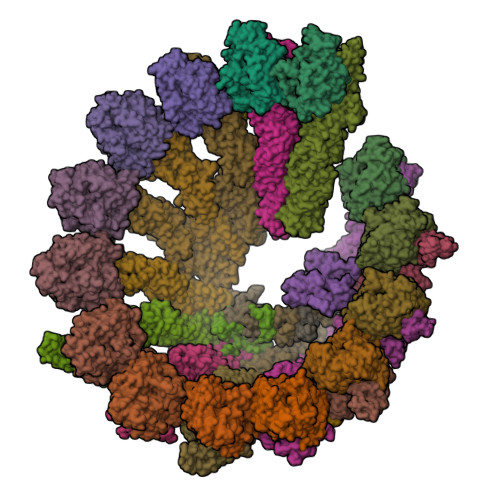
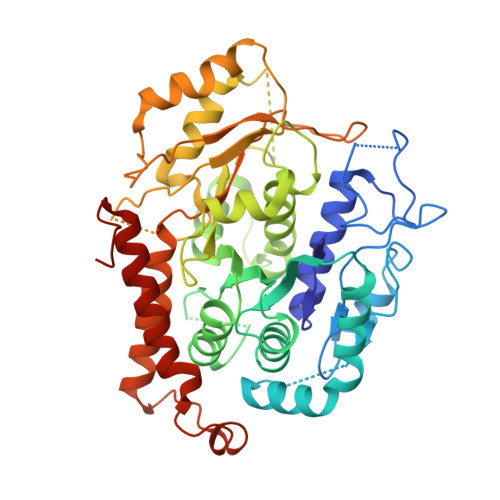
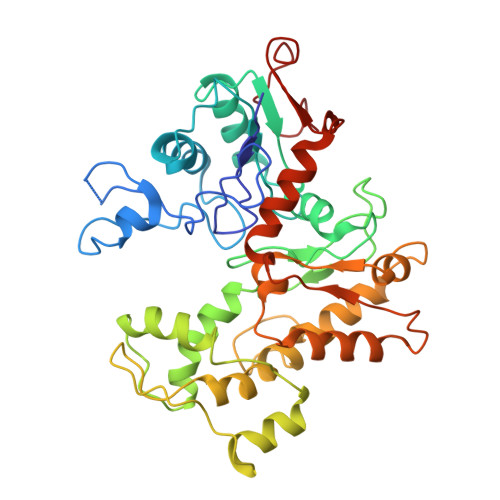
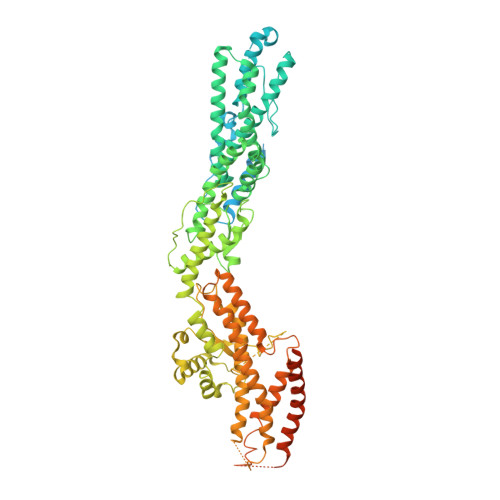
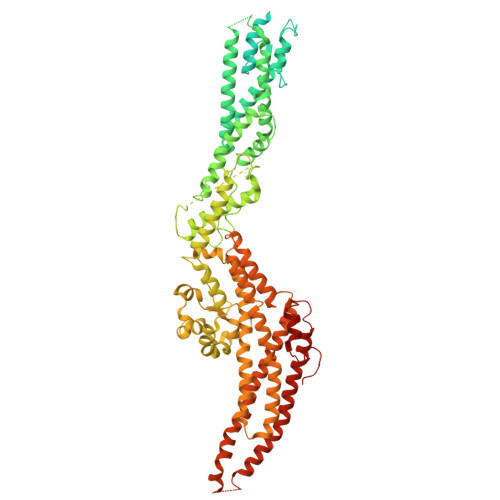
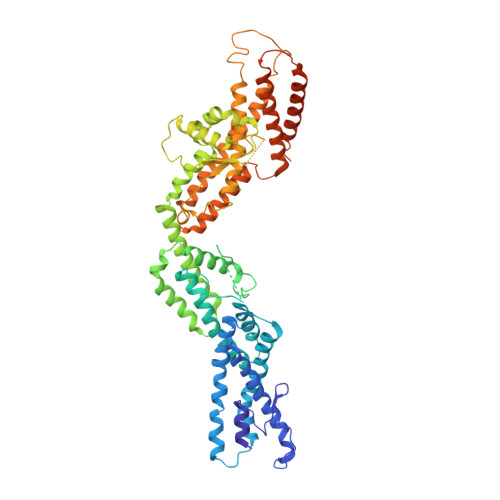
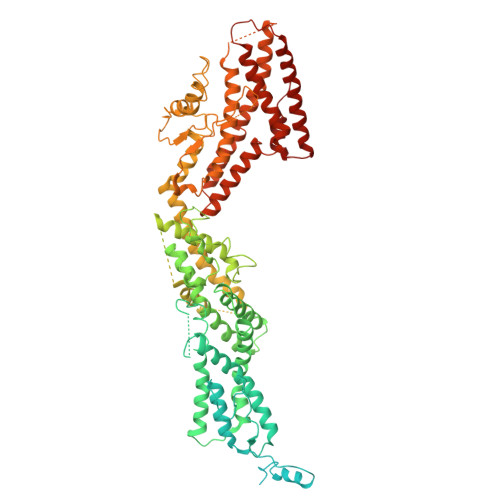
CDK5RAP2 activates microtubule nucleator gamma TuRC by facilitating template formation and actin release.
Serna, M., Zimmermann, F., Vineethakumari, C., Gonzalez-Rodriguez, N., Llorca, O., Luders, J.(2024) Dev Cell 59: 3175
- PubMed: 39321809
- DOI: https://doi.org/10.1016/j.devcel.2024.09.001
- Primary Citation of Related Structures:
8RX1 - PubMed Abstract:
To organize microtubules, cells tightly control the activity of the microtubule nucleator γ-tubulin ring complex (γTuRC). The open ring-shaped γTuRC was proposed to nucleate microtubules by a template mechanism. However, its splayed structure does not match microtubule symmetry, leaving it unclear how γTuRC becomes an efficient nucleator. Here, we identify the mechanism of γTuRC activation by CDK5RAP2 centrosomin motif 1 (CM1). Using cryoelectron microscopy (cryo-EM), we find that activation involves binding of multiple CM1 dimers to five distinct sites around the outside of the γTuRC cone, which crucially depends on regulatory modules formed by MZT2 and the N-terminal extensions of GCP2 subunits. CM1 binding promotes lateral interactions between GCP subunits to facilitate microtubule-like conformations and release of luminal actin that is integral to non-activated γTuRC. We propose a model where generation of γTuRC with an expanded conformational range, rather than perfect symmetry, is sufficient to boost nucleation activity.
Organizational Affiliation:
Structural Biology Programme, Spanish National Cancer Research Centre (CNIO), Melchor Fernández Almagro 3, 28029 Madrid, Spain.