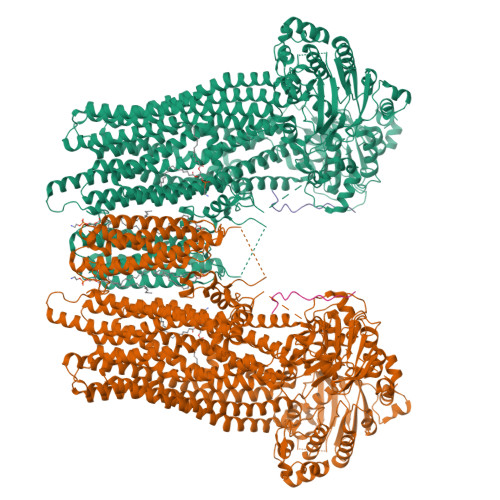
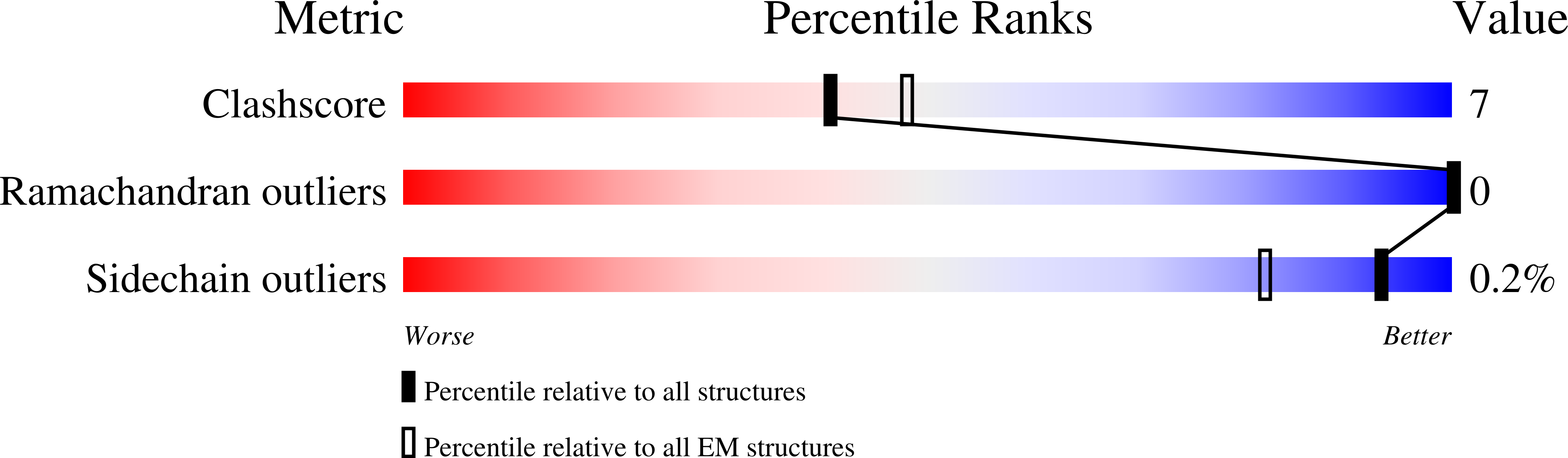Structure of a dimeric full-length ABC transporter.
Bickers, S.C., Benlekbir, S., Rubinstein, J.L., Kanelis, V.(2024) Nat Commun 15: 9946-9946
- PubMed: 39550367
- DOI: https://doi.org/10.1038/s41467-024-54147-8
- Primary Citation of Related Structures:
8SQ0, 8SQM - PubMed Abstract:
Activities of ATP binding cassette (ABC) proteins are regulated by multiple mechanisms, including protein interactions, phosphorylation, proteolytic processing, and/or oligomerization of the ABC protein itself. Here we present the structure of yeast cadmium factor 1 (Ycf1p) in its mature form following cleavage by Pep4p protease. Ycf1p, a C subfamily ABC protein (ABCC), is homologue of human multidrug resistance protein 1. Remarkably, a portion of cleaved Ycf1p forms a well-ordered dimer, alongside monomeric particles also present in solution. While numerous other ABC proteins have been proposed to dimerize, no high-resolution structures have been reported. Both phosphorylation of the regulatory (R) region and ATPase activity are lower in the Ycf1p dimer compared to the monomer, indicating that dimerization affects Ycf1p function. The interface between Ycf1p protomers features protein-protein interactions and contains bound lipids, suggesting that lipids stabilize the dimer. The Ycf1p dimer structure may inform the dimerization interfaces of other ABCC dimers.
Organizational Affiliation:
Department of Chemistry, University of Toronto, Toronto, ON, Canada.