Nipah and Hendra Virus use an adjustable latch in Receptor Engagement
von Itzstein, M.S., Winger, M., Malde, A.K., Holt, S., McAtamney, S., Hartley-Tassell, L., Ve, T., Maggioni, A., von Itzstein, M.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glycoprotein G | 453 | Henipavirus hendraense | Mutation(s): 0 | 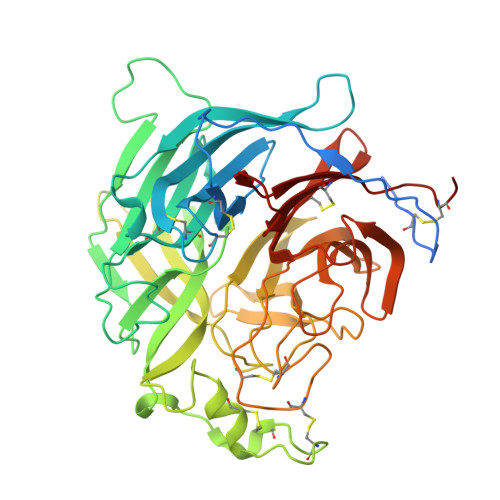 | |
UniProt | |||||
Find proteins for O89343 (Hendra virus (isolate Horse/Autralia/Hendra/1994)) Explore O89343 Go to UniProtKB: O89343 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O89343 | ||||
Glycosylation | |||||
| Glycosylation Sites: 3 | |||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | E [auth A], F [auth A], G [auth B] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 55.822 | α = 90 |
| b = 80.155 | β = 106.483 |
| c = 95.136 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Health and Medical Research Council (NHMRC, Australia) | Australia | APP1047824 |
| Australian Research Council (ARC) | Australia | DP1094549 |
| Australian Research Council (ARC) | Australia | DE17010078 |