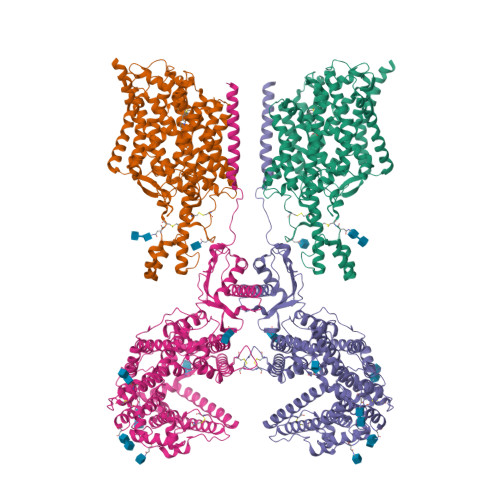
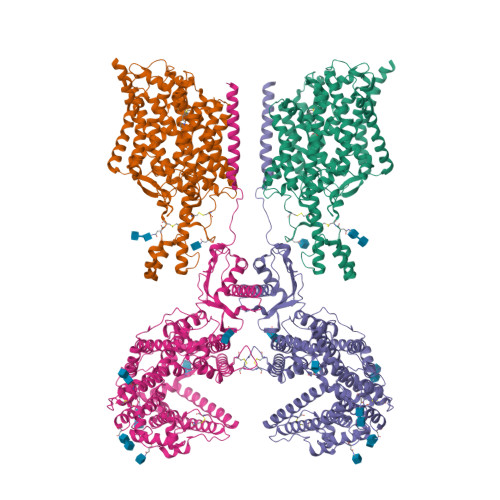
Cryo-EM structure of ACE2-SIT1 in complex with tiagabine.
Broer, A., Hu, Z., Kukulowicz, J., Yadav, A., Zhang, T., Dai, L., Bajda, M., Yan, R., Broer, S.(2024) J Biological Chem 300: 107687-107687
- PubMed: 39159813
- DOI: https://doi.org/10.1016/j.jbc.2024.107687
- Primary Citation of Related Structures:
8WM3 - PubMed Abstract:
The pharmacology of amino acid transporters in the SLC6 family is poorly developed compared to that of the neurotransmitter transporters. To identify new inhibitors of the proline transporter SIT1 (SLC6A20), its expression in Xenopus laevis oocytes was optimized. Trafficking of SIT1 was augmented by co-expression of angiotensin-converting enzyme 2 (ACE2) in oocytes but there was no strict requirement for co-expression of ACE2. A pharmacophore-guided screen identified tiagabine as a potent non-competitive inhibitor of SIT1. To understand its binding mode, we determined the cryo-electron microscopy (cryo-EM) structure of ACE2-SIT1 bound with tiagabine. The inhibitor binds close to the orthosteric proline binding site, but due to its size extends into the cytosolic vestibule. This causes the transporter to adopt an inward-open conformation, in which the intracellular gate is blocked. This study provides the first structural insight into inhibition of SIT1 and generates tools for a better understanding of the ACE2-SIT1 complex. These findings may have significance for SARS-CoV-2 binding to its receptor ACE2 in human lung alveolar cells where SIT1 and ACE2 are functionally expressed.
Organizational Affiliation:
Research School of Biology, Australian National University, Canberra, Australia.