Unravelling nicotinic receptor and ligand features underlying neonicotinoid knockdown actions on the malaria vector mosquito Anopheles gambiae.
Ito, R., Kamiya, M., Takayama, K., Mori, S., Matsumoto, R., Takebayashi, M., Ojima, H., Fujimura, S., Yamamoto, H., Ohno, M., Ihara, M., Okajima, T., Yamashita, A., Colman, F., Lycett, G.J., Sattelle, D.B., Matsuda, K.(2024) Open Biol 14: 240057-240057
- PubMed: 39043224
- DOI: https://doi.org/10.1098/rsob.240057
- Primary Citation of Related Structures:
8XSU - PubMed Abstract:
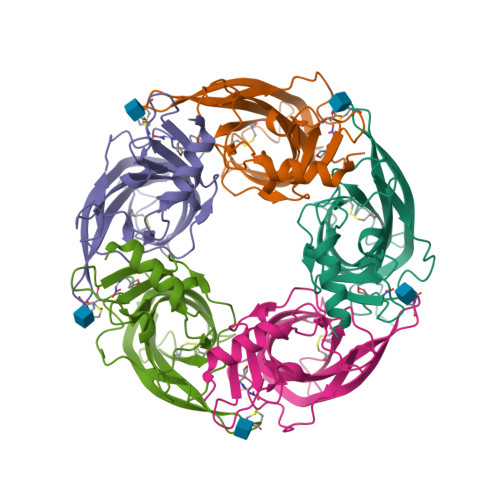
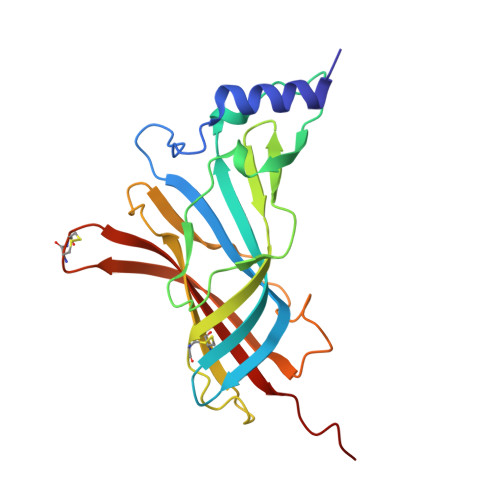
With the spread of resistance to long-established insecticides targeting Anopheles malaria vectors, understanding the actions of compounds newly identified for vector control is essential. With new commercial vector-control products containing neonicotinoids under development, we investigate the actions of 6 neonicotinoids (imidacloprid, thiacloprid, clothianidin, dinotefuran, nitenpyram and acetamiprid) on 13 Anopheles gambiae nicotinic acetylcholine receptor (nAChR) subtypes produced by expression of combinations of the Ag α 1, Ag α 2, Ag α 3, Ag α 8 and Ag β 1 subunits in Xenopus laevis oocytes, the Drosophila melanogaster orthologues of which we have previously shown to be important in neonicotinoid actions. The presence of the Ag α 2 subunit reduces neonicotinoid affinity for the mosquito nAChRs, whereas the Ag α 3 subunit increases it. Crystal structures of the acetylcholine binding protein (AChBP), an established surrogate for the ligand-binding domain, with dinotefuran bound, shows a unique target site interaction through hydrogen bond formation and CH-N interaction at the tetrahydrofuran ring. This is of interest as dinotefuran is also under trial as the toxic element in baited traps. Multiple regression analyses show a correlation between the efficacy of neonicotinoids for the Ag α 1/Ag α 2/Ag α 8/Ag β 1 nAChR, their hydrophobicity and their rate of knockdown of adult female An. gambiae , providing new insights into neonicotinoid features important for malaria vector control.
Organizational Affiliation:
Department of Applied Biological Chemistry, Faculty of Agriculture, Kindai University, 3327-204 Nakamachi, Nara 631-8505, Japan.