Biophysical characterization of blood coagulation factor VIII binding to lipid nanodiscs that mimic activated platelet surfaces.
Avery, N.G., Young, I.R., Lu, S., Vaughan, J.D., Korus, P.S., Richardson, T.N., Childers, K.C., Smirnov, S.L., Spiegel Jr., P.C.(2024) J Thromb Haemost
- PubMed: 39549835
- DOI: https://doi.org/10.1016/j.jtha.2024.11.003
- Primary Citation of Related Structures:
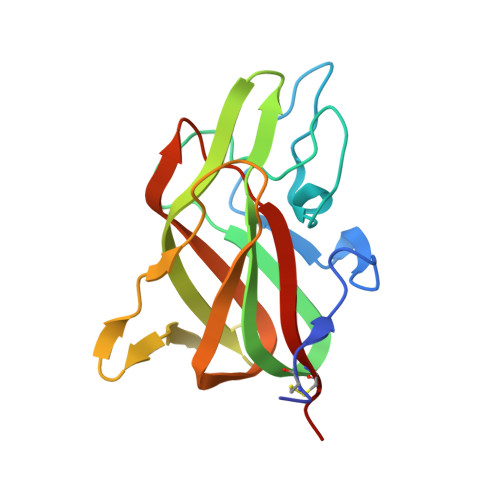
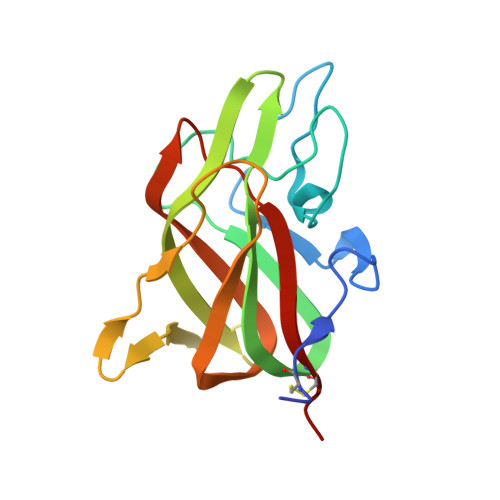
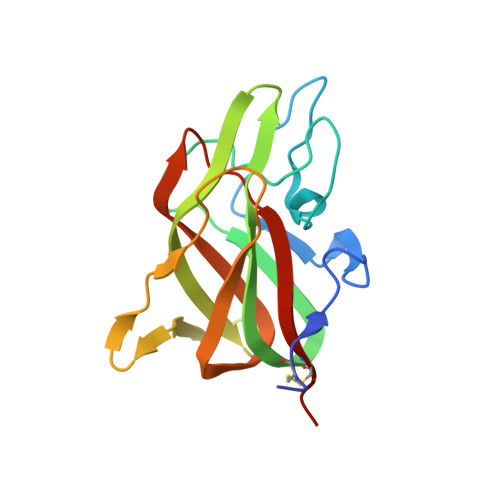
9D5D - PubMed Abstract:
Following proteolytic activation, activated blood coagulation factor (F)VIII (FVIIIa) binds to activated platelet membranes, forming the intrinsic tenase complex with activated FIX (FIXa). Previous studies have identified the C1 and C2 domains as the membrane binding domains of FVIII through conserved arginine residues. A membrane binding model for the FVIII C domains proposes that surface-exposed hydrophobic and positively charged residues at each C domain interact with the membrane, yet a comprehensive thermodynamic and structural description of this interaction is lacking. To determine residues of interaction, thermodynamics, and membrane binding preference for FVIII membrane association. The binding of FVIII constructs to lipid nanodiscs was characterized by nuclear magnetic resonance, isothermal titration calorimetry, bio-layer interferometry, and X-ray crystallography. The thermodynamics of FVIII membrane binding indicated that the C1 domain associates through an enthalpically driven process while the C2 domain is entropically driven. Alanine mutations to surface-exposed hydrophobic residues in the C2 domain revealed differential effects on membrane binding, highlighting important determinants at the residue level. The structure of a C2 double mutant, L2251A/L2252A, demonstrated that its decreased affinity is likely due to decreasing the surface area hydrophobicity. Nuclear magnetic resonance studies with the C2 domain identified residues of interaction with soluble O-phospho-L-serine as well as lipid nanodiscs. Lastly, increasing phosphatidylethanolamine and decreasing phosphatidylserine content decreased overall FVIII affinity for membrane surfaces. This study provides further insight into the molecular basis for how FVIII interacts with platelets to form the intrinsic tenase complex.
Organizational Affiliation:
Chemistry Department, Western Washington University, Bellingham, Washington, USA.