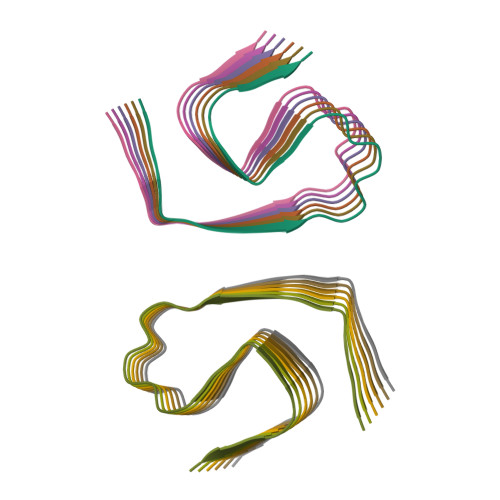Impacts of D-aspartate on the Aggregation Kinetics and Structural Polymorphism of Amyloid beta Peptide 1-42.
Hsiao, L.C., Lee, C.H., Mazmanian, K., Yoshida, M., Ito, G., Murata, T., Utsunomiya-Tate, N., Haino, T., Tate, S.I., Hsu, S.D.(2025) J Mol Biology 437: 169092-169092
- PubMed: 40090459
- DOI: https://doi.org/10.1016/j.jmb.2025.169092
- Primary Citation of Related Structures:
9JAZ, 9JB0, 9JB1, 9JB2 - PubMed Abstract:
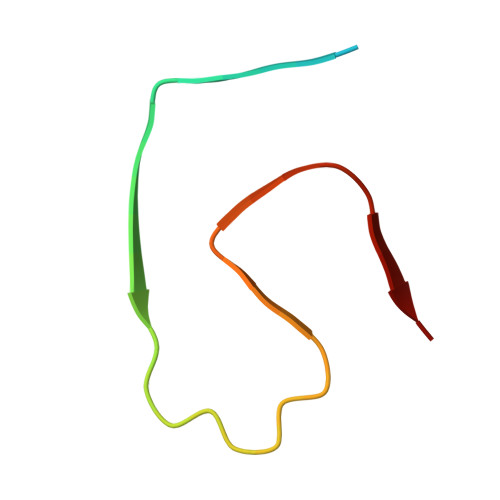
Isomerization of L-Aspartate (L-Asp) into D-aspartate (D-Asp) occurs naturally in proteins at a rate that is much faster than that of other amino acid types. Accumulation of D-Asp is age-dependent, which could alter protein structures and, therefore, functions. Site-specific introduction of D-Asp can accelerate aggregation kinetics of a variety of proteins associated with misfolding diseases. Here, we showed by thioflavin T fluorescence that the isomerization of L-Asp at different positions of amyloid β peptide 1-42 (Aβ42) generates opposing effects on its aggregation kinetics. We further determined the atomic structures of Aβ42 amyloid fibrils harboring a single D-Asp at position 23 and two D-Asp at positions 7 and 23 by cryo-electron microscopy helical reconstruction - cross-validated by cryo-electron tomography and atomic force microscopy - to reveal how D-Asp 7 contributes to the formation of a unique triple stranded amyloid fibril structure stabilized by two threads of well-ordered water molecules. These findings provide crucial insights into how the conversion from L- to D-Asp influences the aggregation propensity and amyloid polymorphism of Aβ42.
Organizational Affiliation:
Institute of Biological Chemistry, Academia Sinica, Taipei 11529, Taiwan; Institute of Biochemical Sciences, National Taiwan University, Taipei 10617, Taiwan.