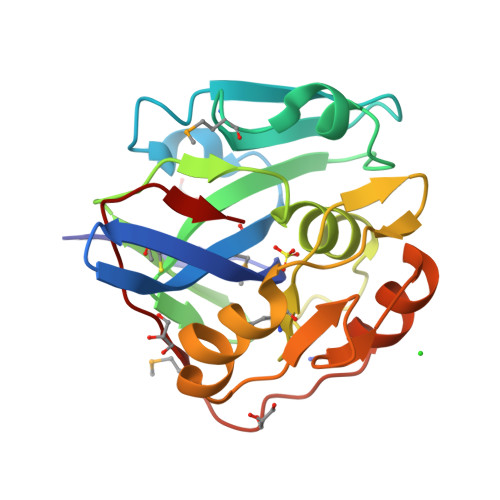
Crystal Structure of a Viral Protease Intramolecular Acyl-enzyme Complex: INSIGHTS INTO cis-CLEAVAGE AT THE VP4/VP3 JUNCTION OF TELLINA BIRNAVIRUS.
Chung, I.Y., Paetzel, M.(2011) J Biological Chem 286: 12475-12482
- PubMed: 21288899
- DOI: https://doi.org/10.1074/jbc.M110.198812
- Primary Citation of Related Structures:
3P06 - PubMed Abstract:
Viruses of the Birnaviridae family are characterized by their bisegmented double-stranded RNA genome that resides within a single-shelled non-enveloped icosahedral particle. They infect birds, aquatic organisms, and insects. Tellina virus 1 (TV-1) is an Aquabirnavirus isolated from the mollusk Tellina tenuis. It encodes a polyprotein (NH2-pVP2-X-VP4-VP3-COOH) that is cleaved by the self-encoded protease VP4 to yield capsid precursor protein pVP2, peptide X, and ribonucleoprotein VP3. Here we report the crystal structure of an intramolecular (cis) acyl-enzyme complex of TV-1 VP4 at 2.1-Å resolution. The structure reveals how the enzyme can recognize its own carboxyl terminus during the VP4/VP3 cleavage event. The methyl side chains of Ala830(P1) and Ala828(P3) at the VP4/VP3 junction point into complementary shallow and hydrophobic S1 and S3 binding pockets adjacent to the VP4 catalytic residues: nucleophile Ser738 and general base Lys777. The electron density clearly shows that the carbonyl carbon of Ala830 is covalently attached via an ester bond to the Oγ of Ser738. A highly ordered water molecule in the active site is coordinated in the proper position to act as the deacylating water. A comparative analysis of this intramolecular (cis) acyl-enzyme structure with the previously solved intermolecular (trans) acyl-enzyme structure of infectious pancreatic necrosis virus VP4 explains the narrower specificity observed in the cleavage sites of TV-1 VP4.
Organizational Affiliation:
Department of Molecular Biology and Biochemistry, Simon Fraser University, Burnaby, British Columbia V5A 1S6, Canada.